‘1000 genomes barrier’ broken
November 1, 2012
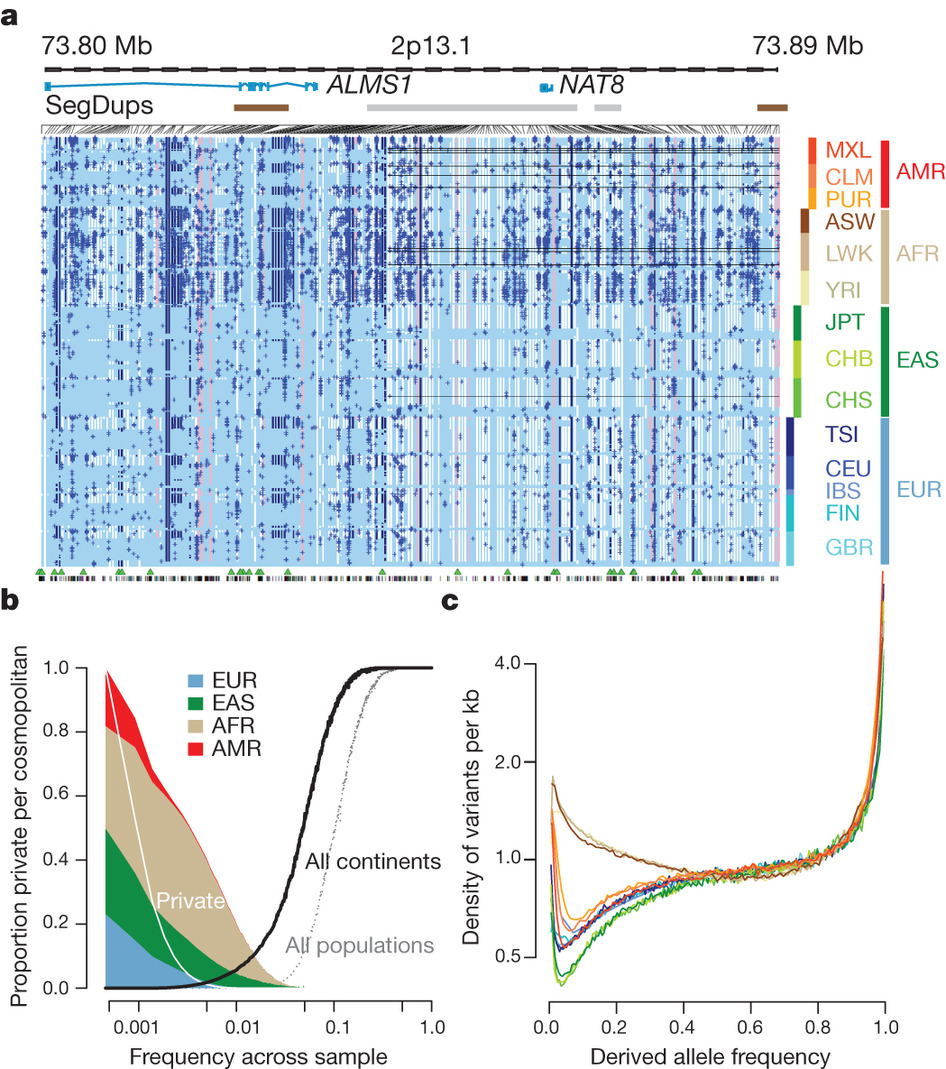
The distribution of rare and common variants (credit: /Nature)
A landmark project that has sequenced 1,092 human genomes from individuals around the world will help researchers to interpret the genetic changes in people with disease.
This first study to break the “1000 genomes barrier” will enable scientists to begin to examine genetic variations at the scale of the populations of individual countries, as well as guiding them in their search for the rare genetic variations related to many diseases.
The vast majority of genetic variation is shared with populations around the world, but it is thought that a lot of the contribution to disease may come from rare variants of genes, found in 1 in 100 people or fewer.
Researchers need to find these rare variants to see who has them and work out how they might contribute to a range of conditions from multiple sclerosis to heart disease and cancer.
The international team behind the 1000 Genomes Project found that rare gene variants tend to be restricted to particular geographic regions, because they typically arise from more recent mutations since humans spread across the world.
By drilling down to genetic variants occurring at the scale of 1 in 100 people for the first time, this study will enable researchers to interpret an individual’s genome in the context of the genetic variation found in their own national population. This will help identify differences between genomes from 14 countries from Europe (including the UK), the Americas, East Asia and Africa.
“We are all walking natural experiments; some of our genes are switched off, some are active, whilst others are overactive,” said Professor Gil McVean of Oxford University‘s Department of Statistics and Wellcome Trust Centre for Human Genetics, the lead author for the study. “Our research has found that each apparently healthy person carries hundreds of rare variants of genes that have a significant impact on how genes work, and a handful (from two to five) of rare changes that have been identified as contributing to disease in other people.”
The study has been designed so that, as well as the genome data, researchers have access to living cells (cell lines) from all 1,092 of the individuals whose genomes have been sequenced. Scientists can now study how differences in the biology of these cells correlate with genetic differences.
“There are variations that jump out from the data as looking ‘a bit bad for you,’ for example mutations in regions that regulate genes are likely to be ‘bad news’ — possibly doing something dramatic to how cells behave,” said Dr Richard Durbin from the Wellcome Trust Sanger Institute, co-chair of the 1000 Genomes Project. “Using our data you can now look to see if natural selection has been getting rid of such mutations — giving you a clue as to how harmful these variants might be.”
The team’s work is already being used to screen cancer genomes for mutations that might identify therapeutic pathways, to interpret the genomes of children with developmental disorders and to pin-point variation that leads to increased risk for complex diseases such as heart disease or multiple sclerosis.
Professor McVean said: “Our research shows that you can take localism much further: for example, even just within the UK, Orkney islanders will have different variations from mainlanders, and will be different again from those from other nearby islands. In the future we would like to reach the scale of having a grid of individuals giving us a different genome every couple of square kilometres but there is a long way to go before we can make this a reality.”
Sir Mark Walport, Director of the Wellcome Trust, which part-funded the study, said: “It is quite remarkable that we have gone from completion of the first human genome sequence in 2003 to being able to sequence more than a 1000 human genomes for a single study in 2012. This study is an important contribution to our understanding of human genetic variation in health and disease and the DNA sequences are freely available for analysis and use by researchers.”