New imaging method developed at Stanford reveals stunning details of brain connections
November 18, 2010
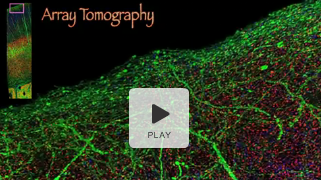
Visual reconstruction (from array-tomography data) of synapses in the mouse somatosensory cortex, which is responsive to whisker stimulation. Neurons are depicted in green. Multicolored dots represent separate synapses. (Stanford University Medical Center)
Researchers at the Stanford University School of Medicine, applying a state-of-the-art imaging system to brain-tissue samples from mice, have been able to quickly and accurately locate and count the myriad connections between nerve cells in unprecedented detail, as well as to capture and catalog those connections’ surprising variety.
A typical healthy human brain contains about 200 billion neurons, linked to one another via hundreds of trillions of synapses. One neuron may make as many as tens of thousands of synaptic contacts with other neurons, said Stephen Smith, PhD, professor of molecular and cellular physiology and senior author of a paper describing the study, published Nov. 18 in Neuron.
Because synapses are so minute and packed so closely together, it has been hard to get a handle on the complex neuronal circuits that do our thinking, feeling and activation of movement.The new method works by combining high-resolution photography with specialized fluorescent molecules that bind to different proteins and glow in different colors. Massive computing power captures this information and converts it into imagery.
Examined up close, a synapse — less than a thousandth of a millimeter in diameter — is a specialized interface consisting of the edges of two neurons, separated by a tiny gap. Chemicals squirted out of the edge of one neuron diffuse across the gap, triggering electrical activity in the next and thus relaying a nervous signal. There are perhaps a dozen known types of synapses, categorized according to the kind of chemical employed in them. Different synaptic types differ correspondingly in the local proteins, on one abutting neuron or the other, that are associated with the packing, secretion and uptake of the different chemicals.
Synapse numbers in the brain vary over time. Periods of massive proliferation in fetal development, infancy and adolescence give way to equally massive bursts of “pruning” during which underused synapses are eliminated, and eventually to a steady, gradual decline with increasing age. The number and strength of synaptic connections in various brain circuits also fluctuate with waking and sleeping cycles, as well as with learning. Many neurodegenerative disorders are marked by pronounced depletion of specific types of synapses in key brain regions.
In particular, the cerebral cortex — a thin layer of tissue on the brain’s surface — is a thicket of prolifically branching neurons. “In a human, there are more than 125 trillion synapses just in the cerebral cortex alone,” said Smith. That’s roughly equal to the number of stars in 1,500 Milky Way galaxies, he noted.
Synapses in the brain are crowded in so close together that they cannot be reliably resolved by even the best of traditional light microscopes, Smith said. “Now we can actually count them and, in the bargain, catalog each of them according to its type.”
Array tomography, an imaging method co-invented by Smith and Kristina Micheva, PhD, who is a senior staff scientist in Smith’s lab, was used in this study as follows: A slab of tissue — in this case, from a mouse’s cerebral cortex — was carefully sliced into sections only 70 nanometers thick. These ultrathin sections were stained with antibodies designed to match 17 different synapse-associated proteins, and they were further modified by conjugation to molecules that respond to light by glowing in different colors.
The antibodies were applied in groups of three to the brain sections. After each application huge numbers of extremely high-resolution photographs were automatically generated to record the locations of different fluorescing colors associated with antibodies to different synaptic proteins. The antibodies were then chemically rinsed away and the procedure was repeated with the next set of three antibodies, and so forth. Each individual synapse thus acquired its own protein-composition “signature,” enabling the compilation of a very fine-grained catalog of the brain’s varied synaptic types.
All the information captured in the photos was recorded and processed by novel computational software, most of it designed by study co-author Brad Busse, a graduate student in Smith’s lab. It virtually stitched together all the slices in the original slab into a three-dimensional image that can be rotated, penetrated and navigated by the researchers.
The Stanford team used brain samples from a mouse that had been bioengineered so that particularly large neurons that abound in the cerebral cortex express a fluorescent protein, normally found in jellyfish, that glows yellowish-green. This let them visualize synapses against the background of the neurons they linked.
The researchers were able to “travel” through the resulting 3-D mosaic and observe different colors corresponding to different synaptic types just as a voyager might transit outer space and note the different hues of the stars dotting the infinite blackness. A movie was also created by this software.
This level of detailed visualization has never been achieved before, Smith said. “The entire anatomical context of the synapses is preserved. You know right where each one is, and what kind it is,” he said.
Observed in this manner, the brain’s overall complexity is almost beyond belief, said Smith. “One synapse, by itself, is more like a microprocessor —with both memory-storage and information-processing elements — than a mere on/off switch. In fact, one synapse may contain on the order of 1,000 molecular-scale switches. A single human brain has more switches than all the computers and routers and Internet connections on Earth,” he said.
In the course of the study, whose primary purpose was to showcase the new technique’s application to neuroscience, Smith and his colleagues discovered some novel, fine distinctions within a class of synapses previously assumed to be identical. His group is now focused on using array tomography to tease out more such distinctions, which should accelerate neuroscientists’ progress in, for example, identifying how many of which subtypes are gained or lost during the learning process, after an experience such as traumatic pain, or in neurodegenerative disorders such as Alzheimer’s. With support from the National Institutes of Health, Smith’s lab is using array tomography to examine tissue samples from Alzheimer’s brains obtained from Stanford and the University of Pennsylvania.
“I anticipate that within a few years, array tomography will have become an important mainline clinical pathology technique, and a drug-research tool,” Smith said. He and Micheva are founding a company that is now gathering investor funding for further work along these lines. Stanford’s Office of Technology Licensing has obtained one U.S. patent on array tomography and filed for a second.
The Neuron study was funded by the NIH, the Gatsby Charitable Trust, the Howard Hughes Medical Institute, Stanford’s Bio-X program and a gift from Lubert Stryer, MD, the emeritus Mrs. George A. Winzer Professor of Cell Biology in the medical school’s Department of Neurobiology. Other Stanford co-authors of the paper were neuroscience graduate student Nicholas Weiler and senior research scientist Nancy O’Rourke, PhD.
Information about the school’s Departments of Neurosurgery and of Neurology and Neurological Science, which also supported the work, is available at http://med.stanford.edu/neurosurgery and http://neurology.stanford.edu.
- Adapted from materials provided by Stanford University Medical Center