A battery made of wood: long-lasting, efficient, environmentally friendly
June 23, 2013
Wood fibers can withstand the swelling and shrinking in charging/discharging batteries (credit: University of Maryland)
University of Maryland researchers have developed and tested a battery with anodes made of tin-coated wood that are a thousand times thinner than a piece of paper.
Using sodium instead of lithium (which is used in many rechargeable batteries) makes the battery environmentally benign. Also, while sodium doesn’t store energy as efficiently as lithium, its low cost and use of commonly available materials would make a sodium battery ideal to store huge amounts of energy, such as solar energy at a electrical power plant.
Existing batteries are often created on stiff anode bases, which are too brittle to withstand the swelling and shrinking that happens as electrons are stored in the battery and used up. The scientists found that wood fibers are supple enough to let their sodium-ion battery last more than 400 charging cycles, which puts it among the longest-lasting nanobatteries.
“The inspiration behind the idea comes from the trees,” said Liangbing Hu, an assistant professor of materials science. “Wood fibers that make up a tree once held mineral-rich water, and so are ideal for storing liquid electrolytes.”
Lead author Hongli Zhu and other team members noticed that after charging and discharging the battery hundreds of times, the wood ended up wrinkled but intact. Computer models showed that that the wrinkles effectively relax the stress in the battery during charging and recharging, so that the battery can survive many cycles.
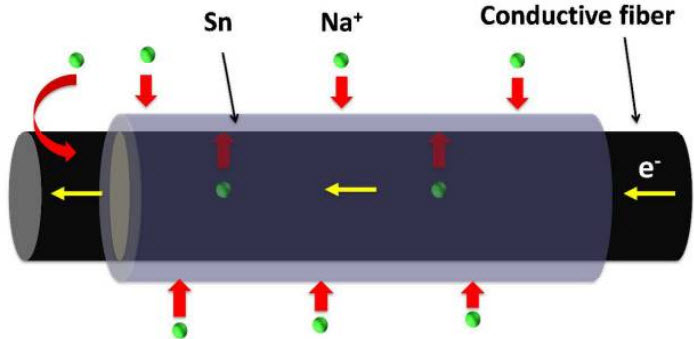
Dual pathways for sodium (Na+) ion transport: through the fiber cell walls and diffusion at the tin (Sn) film surface (credit: University of Maryland)
“Pushing sodium ions through tin anodes in batteries often weakens the tin’s connection to its base material,” said Li, an associate professor of mechanical engineering.
“But the wood fibers are soft enough to serve as a mechanical buffer, and thus can accommodate tin’s changes. This is the key to our long-lasting sodium-ion batteries.”
The team’s research was supported by the University of Maryland and the U.S. National Science Foundation.