A better brain implant: listening to single neurons
November 12, 2012
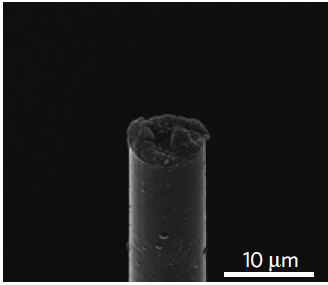
SEM image of a fully assembled, functional microthread electrode (credit: Takashi Kozai)
A thin, flexible electrode developed at the University of Michigan is 10 times smaller than the nearest competition and could make long-term measurements of neural activity practical.
This kind of technology could also be used eventually to send brain-computer-interface (BCI) signals to prosthetic limbs, overcoming inflammation caused by larger electrodes, resulting in damage to both the brain and the electrodes.
Existing electrodes are stiff and enormous compared to neurons. They are also attacked by the immune system, inflaming brain tissue and blocking communication between the electrode and the cells.
The new electrode is unobtrusive. It is a thread of highly conductive carbon fiber, coated in plastic to block out signals from other neurons. The conductive gel pad at the end is compatible with soft cell membranes, and that close connection means the signals from brain cells come in much clearer.
“It’s a huge step forward,” said Nicholas Kotov, the Joseph B. and Florence V. Cejka Professor of Engineering. “This electrode is about seven microns in diameter, or 0.007 millimeters, and its closest competitor is about 25 to 100 microns.”
Listening to single neurons
Artist’s rendering of individual neurons (credit: Takashi Kozai)
To demonstrate how well the electrode listens in on real neurons, the team, headed by Daryl Kipke, professor of biomedical engineering, implanted it into the brains of rats. The electrode’s narrow profile allows it to focus on just one neuron, and the team saw this in the sharp electrical signals coming through the fiber. They weren’t getting a muddle of multiple neurons in conversation.
Listening to single neurons could also help with neuroscience research in general.
“How neurons are communicating with each other? What are the pathways for information processing in the brain? These are the questions that can be answered in the future with this kind of technique,” Kotov said.
“Because these devices are so small, we can combine them with emerging optical techniques to visually observe what the cells are doing in the brain while listening to their electrical signals,” said Takashi Kozai, who led the project as a student in Kipke’s lab and has since earned his Ph.D. “This will unlock new understanding of how the brain works on the cellular and network level.”
The electrode that the team tested is not a clinical trial-ready device, but the results strongly suggest that creating feasible electrode arrays at these small dimensions is a viable path forward for making longer-lasting devices,” he said.
Minimizing immune response and inflammation
In order to listen to a neuron for long, or help people control a prosthetic as they do a natural limb, the electrodes need to be able to survive for years in the brain without doing significant damage. With only six weeks of testing, the team couldn’t say for sure how the electrode would fare in the long term, but the results were promising.
“Typically, we saw a peak in immune response at two weeks, then by three weeks it subsided, and by six weeks it had already stabilized,” Kotov said. “That stabilization is the important observation.”
The rat’s neurons and immune system got used to the electrodes, suggesting that the electronic invaders might be able to stay for the long term.
Kipke is optimistic that prosthetic devices could start linking up with the brain in a decade or so. “The surrounding work of developing very fine robotic control and clinical training protocols — that work is progressing along its own trajectory,” Kipke said.
Kipke is director of the Center for Neural Communication Technology. Kotov, the Joseph B. and Florence V. Cejka Professor of Engineering, is a professor of biomedical engineering, chemical engineering, biomaterials science and engineering, and macromolecular science and engineering. Joerg Lahann, director of the Biointerfaces Institute, is a professor of chemical engineering, materials science and engineering, biomedical engineering, and macromolecular science and engineering.
The work is funded by the National Institutes of Health and the Center for Neural Communication Technology, an NIH-funded biotechnology research center.