A chip that can simulate a tumor’s ‘microenvironment’
September 25, 2014
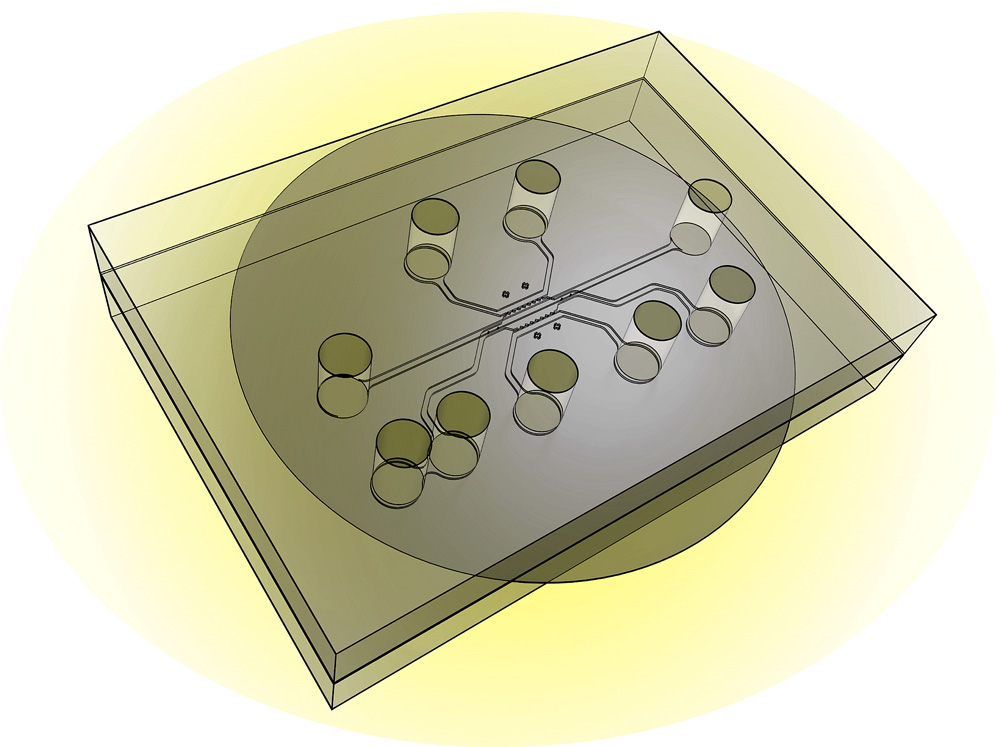
This illustration shows the design of a new chip capable of simulating a tumor’s “microenvironment” to test the effectiveness of nanoparticles and drugs that target cancer. The new system, called a tumor-microenvironment-on-chip device, will allow researchers to study the complex environment surrounding tumors and the barriers that prevent the targeted delivery of therapeutic agents. (Credit: Purdue University photo/Altug Ozcelikkale, Bumsoo Han)
Purdue University researchers have developed a chip capable of simulating a tumor’s “microenvironment” to test the effectiveness of nanoparticles and drugs that target cancer.
The new tumor-microenvironment-on-chip (T-MOC) will allow researchers to study the complex environment surrounding tumors and the barriers that prevent targeted delivery of therapeutic agents, said Bumsoo Han, a Purdue associate professor of mechanical engineering.
Researchers are trying to perfect “targeted delivery” methods using various agents, including an assortment of tiny nanometer-size structures, to selectively attack tumor tissue.
Tumors’ barriers against therapeutic agents
The endothelial cells that make up healthy blood vessels are well organized and have small pores in the tight junctions between them. So one approach is to design nanoparticles that are small enough to pass through pores in the blood vessels surrounding tumors but too large to pass though the pores of vessels in healthy tissue.
The problem: the endothelial cells in blood vessels around tumors are irregular and misshapen, with larger pores in the gaps between the cells.
“It was thought that if nanoparticles were designed to be the right size they could selectively move toward only the tumor,” Han said. But the pressure of “interstitial fluid” inside tumors is greater than that of surrounding healthy tissue. This greater pressure pushes out most drug-delivery and imaging agents, with only a small percentage of them reaching the target tumor.
Navigating the tumor environment
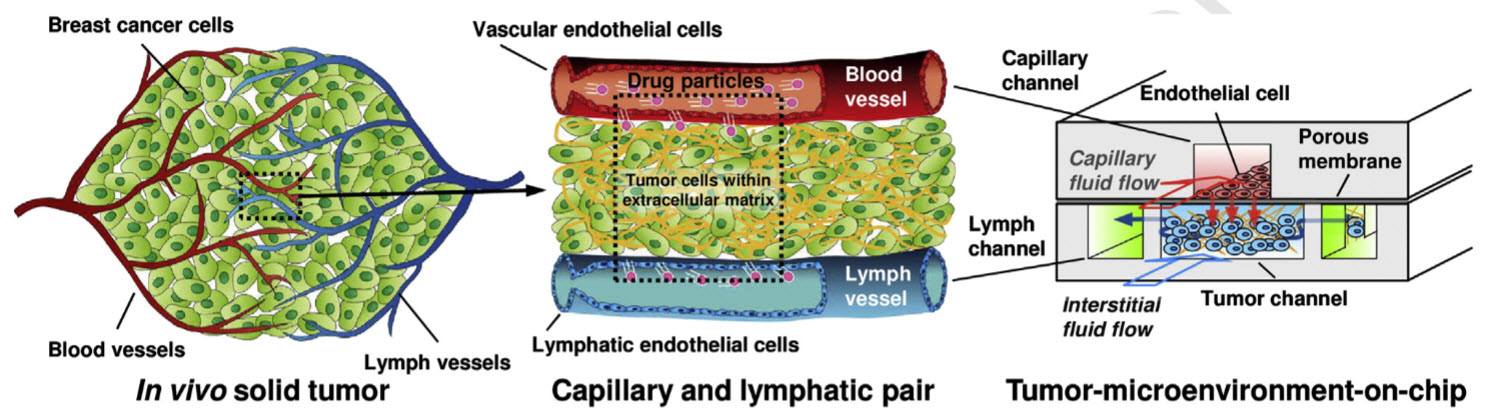
Conceptual design showing how the tumor-microenvironment-on-chip (T-MOC) simulates the complex transport of nanoparticles around a tumor. A functional unit of a solid tumor (a pair of capillary and lymphatic vessels),is mimicked into a 3D microfluidic platform. The top channel (red) simulates the capillary with endothelium (blood vessel lining) on a nanoporous membrane. Nanoparticles to be tested can be introduced along this capillary channel. The bottom layer has a center channel (blue) mimicking a 3D tumor microstructure (cells in 3D matrix) and two side channels simulating the lymphatics (green). (Credit: Bongseop Kwak et al./Journal of Controlled Release)
Now, new research findings suggest that the T-MOC system is capable of simulating the complex environment around tumors and providing detailed information about how nanoparticles move through this environment. Such information could aid efforts to perfect targeted delivery methods.
The findings are detailed in a research paper appearing online this month and will be published in a print edition of the Journal of Controlled Release in November.
The T-MOC chip is about 4.5 centimeters (1.8 inches) square and contains “microfluidic” channels where tumor cells and endothelial cells are cultured. The chip also incorporates extracellular matrix – a spongy, scaffold-like material made of collagen found between cells in living tissue.
The new chip offers an alternative to conventional experimental methods. Studies using cancer cells in petri plates exclude the complex microenvironment surrounding tumors, and research with animals does not show precisely how proposed therapies might work in people.
However, the T-MOC system has the potential to mimic cancer in humans, Han said.
The researchers tested the technology using human breast cancer and endothelial cells and studied how nanoparticles moved within the microenvironment.
Future work will expand to the study of anticancer drugs. Eventually, the devices might be used to grow tumor cells from patients to gauge the effectiveness of specific drugs in those people.
The work was supported by the National Science Foundation, National Institutes of Health, and a Collaboration in Translational Research Award from the Indiana Clinical and Translational Sciences Institute. Han’s work also been supported by the B.S.F. Schaefer Award, the Discovery Park Fellowship, and an Incentive Grant Program from Purdue.
Abstract of Journal of Controlled Release paper
Delivery of therapeutic agents selectively to tumor tissue, which is referred as “targeted delivery,” is one of the most ardently pursued goals of cancer therapy. Recent advances in nanotechnology enable numerous types of nanoparticles (NPs) whose properties can be designed for targeted delivery to tumors. In spite of promising early results, the delivery and therapeutic efficacy of the majority of NPs are still quite limited. This is mainly attributed to the limitation of currently available tumor models to test these NPs and systematically study the effects of complex transport and pathophysiological barriers around the tumors. In this study, thus, we developed a new in vitro tumor model to recapitulate the tumor microenvironment determining the transport around tumors. This model, named tumor-microenvironment-on-chip (T-MOC), consists of 3-dimensional microfluidic channels where tumor cells and endothelial cells are cultured within extracellular matrix under perfusion of interstitial fluid. Using this T-MOC platform, the transport of NPs and its variation due to tumor microenvironmental parameters have been studied including cut-off pore size, interstitial fluid pressure, and tumor tissue microstructure. The results suggest that T-MOC is capable of simulating the complex transport around the tumor, and providing detailed information about NP transport behavior. This finding confirms that NPs should be designed considering their dynamic interactions with tumor microenvironment.