A giant interneuron for ‘sparse coding’
May 16, 2011
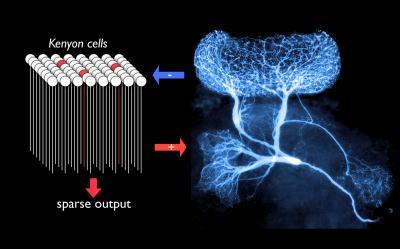
A single "giant" non-spiking, GABAergic interneuron forms an all-to-all negative feedback loop with a population of about 50,000 Kenyon cells, principal neurons of the mushroom bodies. This normalizing feedback loop serves to ensure relatively constant sparseness of mushroom body output across varying input strengths (credit: MPI for Brain Research)
A single giant interneuron tracks in real time the activity of several tens of thousands of neurons in an olfactory center of a locust and feeds inhibition back to all of them to control their collective output, scientists at the Max Planck Institute for Brain Research in Frankfurt have discovered.
The researchers tested how neurons (Kenyon cells) in the insect brain’s mushroom bodies respond with great specificity and extremely rarely. These neurons generally respond with fewer than three electrical impulses when stimulated with the right odor.
This “sparse coding” strategy (where each item is encoded by the strong activation of a relatively small set of neurons) has the advantage that it simplifies the task of storing odor representations in memory. This smooth, graded property enables this giant interneuron to do a kind of real-time, population averaging, thus carrying out an operation that might otherwise require the involvement of hundreds or thousands of individual spiking neurons.
The researchers found that a single GABAergic interneuron forms an “all-to-all” negative feedback loop with a population of about 50,000 Kenyon cells. This normalizing feedback loop serves to ensure relatively constant sparseness of mushroom body output across varying input strengths.
They also found that the giant interneuron can actually turn off the Kenyon cell population completely, and, in turn, is controlled by another inhibitory neuron. This allows the neural network activity to be potentiated or attenuated, and the sensitivity of this feedback loop to be adjusted, the researchers said.
It is very likely that mammals have similar all-to-all control mechanisms in cortical and other circuits. They might not consist of single interneurons, however, but rather of populations of inhibitory neurons with means to couple their responses and actions, the researchers speculate.
Ref: M. Papadopoulou, S. Cassenaer, T. Nowotny, G. Laurent, Normalization for Sparse Encoding of Odors by a Wide-Field Interneuron. Science, 2011; 332 (6030): 721 DOI: 10.1126/science.1201835