A high-efficiency, sustainable process using solar and carbon dioxide to produce methane for natural gas
September 1, 2015
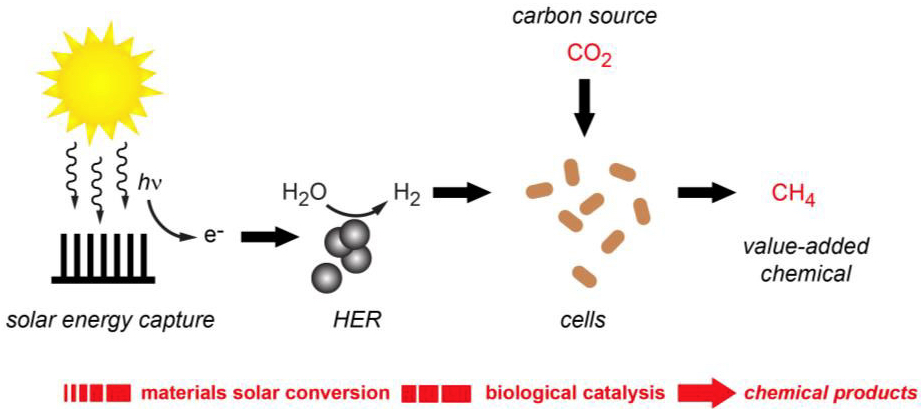
Artificial photosynthesis provides electrical current to produce hydrogen gas from water; the hydrogen then synthesizes carbon dioxide (via microbes) into methane (CH4) (credit: Berkeley Lab)
A team of researchers at the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) has developed a hybrid system that produces hydrogen and uses it (via microbes) to synthesize carbon dioxide into methane, the primary constituent of natural gas.
“We can expect an electrical-to-chemical efficiency of better than 50 percent and a solar-to-chemical energy conversion efficiency of 10 percent if our system is coupled with state-of-art solar panel and electrolyzer,” says Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division and one of the leaders of this study.
“Natural photosynthesis, a solar-to-chemical energy conversion process that combines light, water, and CO2 to make biomass, operates at less than 1% efficiency,” UC Berkeley prof. Chris Chang explained to KurzweilAI. “We have now done a order of magnitude better than nature in this artificial photosynthesis system, albeit in one prototype system where we make methane,” he said. “The advance is that most artificial photosynthesis systems only use light and water, and operate at lower efficiencies to boot. The ability to incorporate CO2 fixation is also a big advance.”
Yang, who also holds appointments with UC Berkeley and the Kavli Energy NanoScience Institute (Kavli-ENSI) at Berkeley, is one of three corresponding authors of a paper describing this research in the Proceedings of the National Academy of Sciences (PNAS).
Sustainable, efficient
Solar energy, a sustainable source of energy, is used to generate hydrogen from water via the hydrogen evolution reaction (HER). The HER is catalyzed by earth-abundant nickel sulfide nanoparticles that operate effectively under biologically compatible conditions.
“Water is the most sustainable starting feedstock for hydrogen,” Chang said. In comparison, “most hydrogen now comes from hydrocarbons, which gives off CO2.”
“We selected methane as an initial target owing to the ease of product separation, the potential for integration into existing infrastructures for the delivery and use of natural gas, and the fact that direct conversion of carbon dioxide to methane with synthetic catalysts has proven to be a formidable challenge,” said Chang.
“Since we still get the majority of our methane from natural gas, a fossil fuel, often from fracking, the ability to generate methane from a renewable hydrogen source (solar) is another important advance.”
Abstract of Hybrid bioinorganic approach to solar-to-chemical conversion
Natural photosynthesis harnesses solar energy to convert CO2 and water to value-added chemical products for sustaining life. We present a hybrid bioinorganic approach to solar-to-chemical conversion in which sustainable electrical and/or solar input drives production of hydrogen from water splitting using biocompatible inorganic catalysts. The hydrogen is then used by living cells as a source of reducing equivalents for conversion of CO2 to the value-added chemical product methane. Using platinum or an earth-abundant substitute, α-NiS, as biocompatible hydrogen evolution reaction (HER) electrocatalysts and Methanosarcina barkeri as a biocatalyst for CO2 fixation, we demonstrate robust and efficient electrochemical CO2 to CH4 conversion at up to 86% overall Faradaic efficiency for ≥7 d. Introduction of indium phosphide photocathodes and titanium dioxide photoanodes affords a fully solar-driven system for methane generation from water and CO2, establishing that compatible inorganic and biological components can synergistically couple light-harvesting and catalytic functions for solar-to-chemical conversion.