A living programmable biocomputing device based on RNA
July 28, 2017
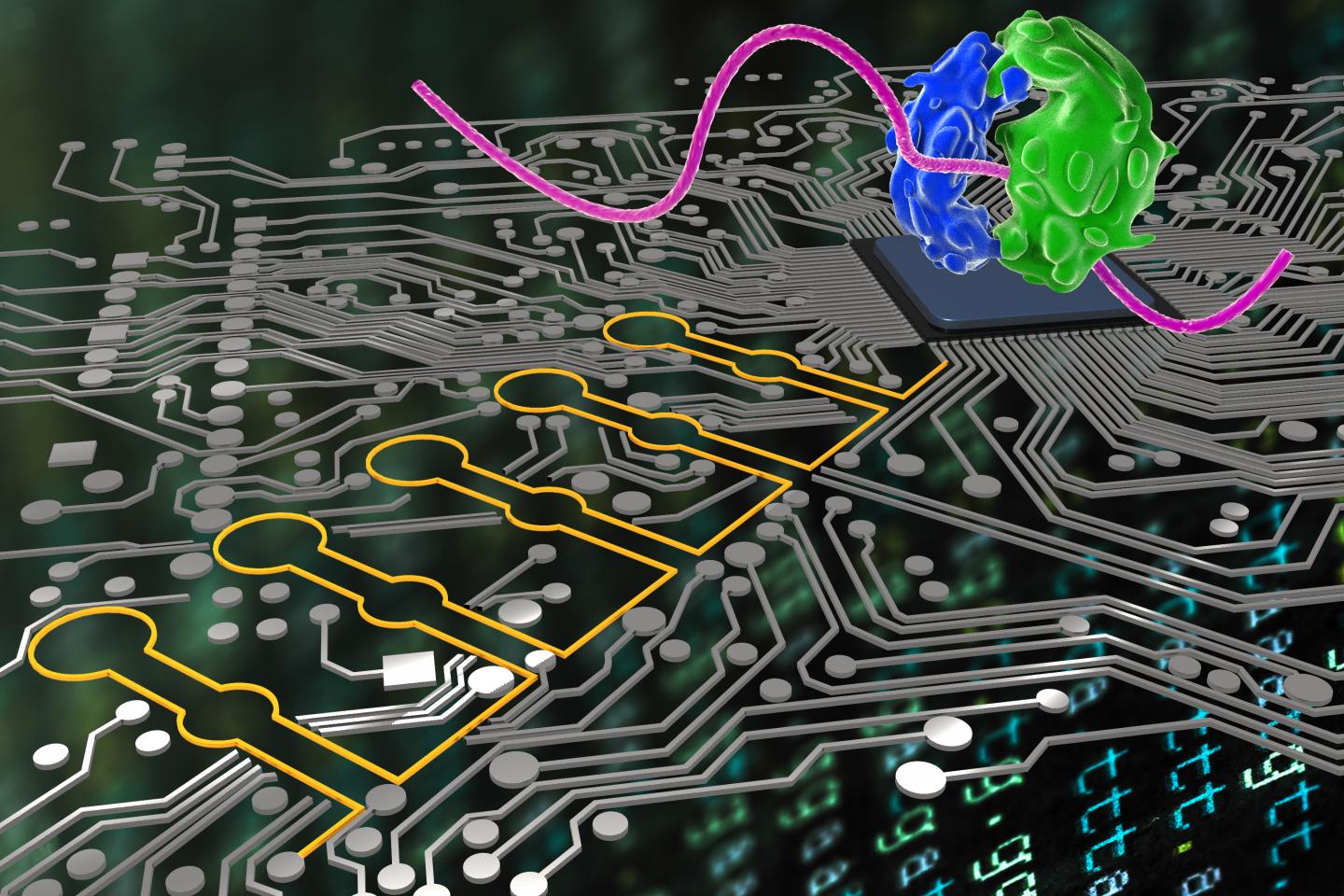
“Ribocomputing devices” ( yellow) developed by a team at the Wyss Institute can now be used by synthetic biologists to sense and interpret multiple signals in cells and logically instruct their ribosomes (blue and green) to produce different proteins. (credit: Wyss Institute at Harvard University)
Synthetic biologists at Harvard’s Wyss Institute for Biologically Inspired Engineering and associates have developed a living programmable “ribocomputing” device based on networks of precisely designed, self-assembling synthetic RNAs (ribonucleic acid). The RNAs can sense multiple biosignals and make logical decisions to control protein production with high precision.
As reported in Nature, the synthetic biological circuits could be used to produce drugs, fine chemicals, and biofuels or detect disease-causing agents and release therapeutic molecules inside the body. The low-cost diagnostic technologies may even lead to nanomachines capable of hunting down cancer cells or switching off aberrant genes.
Biological logic gates
Similar to a digital circuit, these synthetic biological circuits can process information and make logic-guided decisions, using basic logic operations — AND, OR, and NOT. But instead of detecting voltages, the decisions are based on specific chemicals or proteins, such as toxins in the environment, metabolite levels, or inflammatory signals. The specific ribocomputing parts can be readily designed on a computer.
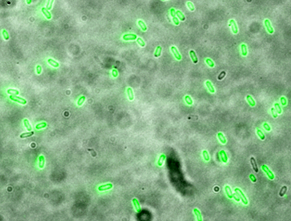
E. coli bacteria engineered to be ribocomputing devices output a green-glowing protein when they detect a specific set of programmed RNA molecules as input signals (credit: Harvard University)
The research was performed with E. coli bacteria, which regulate the expression of a fluorescent (glowing) reporter protein when the bacteria encounter a specific complex set of intra-cellular stimuli. But the researchers believe ribocomputing devices can work with other host organisms or in extracellular settings.
Previous synthetic biological circuits have only been able to sense a handful of signals, giving them an incomplete picture of conditions in the host cell. They are also built out of different types of molecules, such as DNAs, RNAs, and proteins, that must find, bind, and work together to sense and process signals. Identifying molecules that cooperate well with one another is difficult and makes development of new biological circuits a time-consuming and often unpredictable process.
Brain-like neural networks next
Ribocomputing devices could also be freeze-dried on paper, leading to paper-based biological circuits, including diagnostics that can sense and integrate several disease-relevant signals in a clinical sample, the researchers say.
The next stage of research will focus on the use of RNA “toehold” technology* to produce neural networks within living cells — circuits capable of analyzing a range of excitatory and inhibitory inputs, averaging them, and producing an output once a particular threshold of activity is reached. (Similar to how a neuron averages incoming signals from other neurons.)
Ultimately, researchers hope to induce cells to communicate with one another via programmable molecular signals, forming a truly interactive, brain-like network, according to lead author Alex Green, an assistant professor at Arizona State University’s Biodesign Institute.
Wyss Institute Core Faculty member Peng Yin, Ph.D., who led the study, is also Professor of Systems Biology at Harvard Medical School.
The study was funded by the Wyss Institute’s Molecular Robotics Initiative, a Defense Advanced Research Projects Agency (DARPA) Living Foundries grant, and grants from the National Institute of Health (NIH), the Office of Naval Research (ONR), the National Science Foundation (NSF) and the Defense Threat Reduction Agency (DTRA).
* The team’s approach evolved from its previous development of “toehold switches” in 2014 — programmable hairpin-like nano-structures made of RNA. In principle, RNA toehold wwitches can control the production of a specific protein: when a desired complementary “trigger” RNA, which can be part of the cell’s natural RNA repertoire, is present and binds to the toehold switch, the hairpin structure breaks open. Only then will the cell’s ribosomes get access to the RNA and produce the desired protein.
Wyss Institute | Mechanism of the Toehold Switch
Abstract of Complex cellular logic computation using ribocomputing devices
Synthetic biology aims to develop engineering-driven approaches to the programming of cellular functions that could yield transformative technologies. Synthetic gene circuits that combine DNA, protein, and RNA components have demonstrated a range of functions such as bistability, oscillation, feedback, and logic capabilities. However, it remains challenging to scale up these circuits owing to the limited number of designable, orthogonal, high-performance parts, the empirical and often tedious composition rules, and the requirements for substantial resources for encoding and operation. Here, we report a strategy for constructing RNA-only nanodevices to evaluate complex logic in living cells. Our ‘ribocomputing’ systems are composed of de-novo-designed parts and operate through predictable and designable base-pairing rules, allowing the effective in silico design of computing devices with prescribed configurations and functions in complex cellular environments. These devices operate at the post-transcriptional level and use an extended RNA transcript to co-localize all circuit sensing, computation, signal transduction, and output elements in the same self-assembled molecular complex, which reduces diffusion-mediated signal losses, lowers metabolic cost, and improves circuit reliability. We demonstrate that ribocomputing devices in Escherichia coli can evaluate two-input logic with a dynamic range up to 900-fold and scale them to four-input AND, six-input OR, and a complex 12-input expression (A1 AND A2 AND NOT A1*) OR (B1 AND B2 AND NOT B2*) OR (C1 AND C2) OR (D1 AND D2) OR (E1 AND E2). Successful operation of ribocomputing devices based on programmable RNA interactions suggests that systems employing the same design principles could be implemented in other host organisms or in extracellular settings.