A multifunctional medical nanoparticle
September 2, 2014
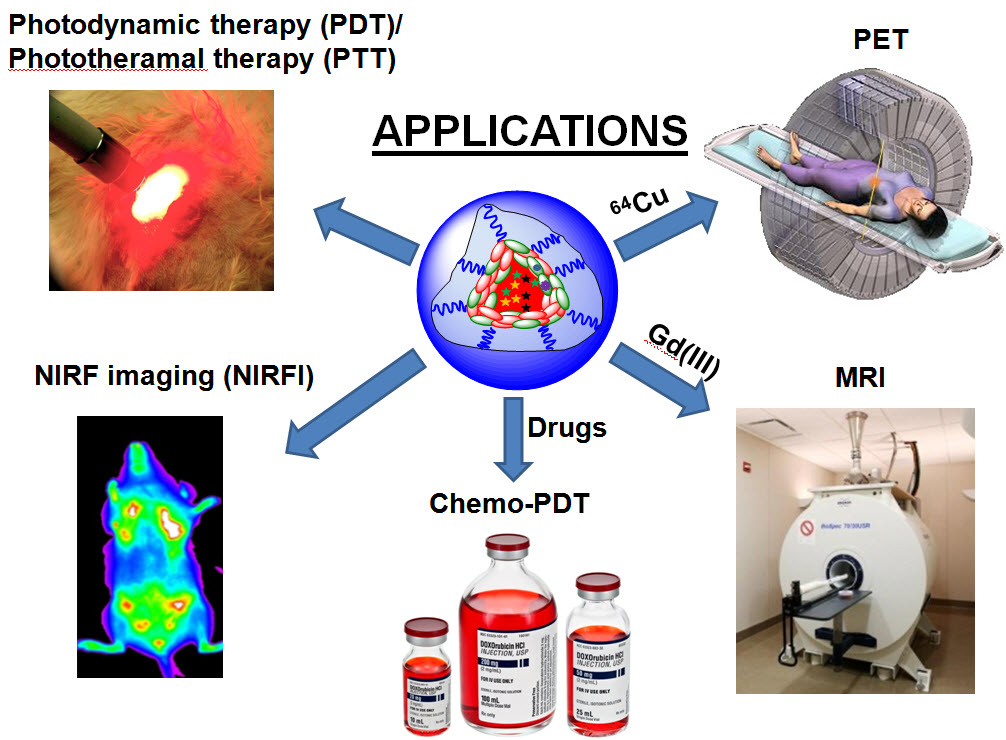
Applications of multifunctional self-assembled nanoparticles (credit: Yuanpei Li et al./Nature Communications)
Researchers at UC Davis Comprehensive Cancer Center and other institutions have created biocompatible multitasking nanoparticles that could be used as contrast agents to light up tumors for MRI and PET scans or deliver chemo and other therapies to destroy tumors. The study was published online in Nature Communications.
“These are amazingly useful particles,” noted co-first author Yuanpei Li, a research faculty member in the Lam laboratory. “As a contrast agent, they make tumors easier to see on MRI and other scans. We can also use them as vehicles to deliver chemotherapy directly to tumors, apply light to make the nanoparticles release singlet oxygen (photodynamic therapy), or use a laser to heat them (photothermal therapy) — all proven ways to destroy tumors.”
Jessica Tucker, program director of Drug and Gene Delivery and Devices at the National Institute of Biomedical Imaging and Bioengineering, which is part of the National Institutes of Health, said the approach outlined in the study has the ability to combine both imaging and therapeutic applications in a single platform, which has been difficult to achieve, especially in an organic, and therefore biocompatible, vehicle.
“This is especially valuable in cancer treatment, where targeted treatment to tumor cells, and the reduction of lethal effects in normal cells, is so critical,” she added.
These are not the first nanoparticles for medical use, but they may be the most versatile. Other particles are good at some tasks but not others. Non-organic particles, such as quantum dots or gold-based materials, work well as diagnostic tools but have safety issues. Organic probes are biocompatible and can deliver drugs but lack imaging or phototherapy applications.
Design of a multifunctional nanoparticle
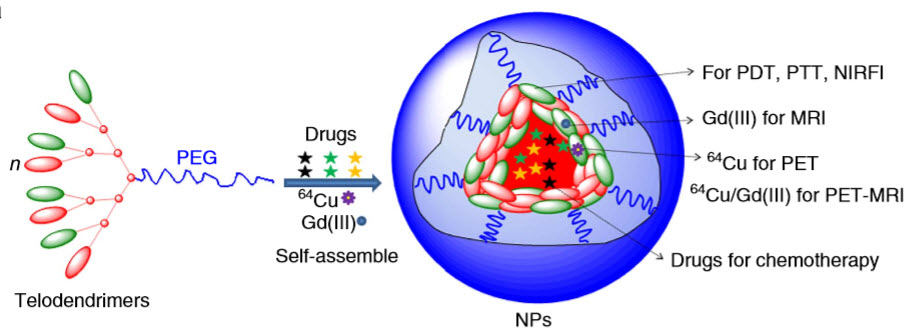
Schematic illustration of construction of a multifunctional nanoparticle (credit: Yuanpei Li et al./Nature Communications)
The nanoparticles are built on a porphyrin/cholic acid polymer and are simple to make. Porphyrins are common organic compounds. Cholic acid is produced by the liver. The basic nanoparticles are 21 nanometers wide (a nanometer is one-billionth of a meter).
To further stabilize the particles, the researchers added the amino acid cysteine (creating CNPs), which prevents them from prematurely releasing their therapeutic payload when exposed to blood proteins and other barriers. At 32 nanometers, CNPs are ideally sized to penetrate tumors, accumulating among cancer cells while sparing healthy tissue.
In the study, the team tested the nanoparticles, both in vitro and in vivo, for a wide range of tasks:
- CNPs effectively transported anti-cancer drugs, such as doxorubicin. Even when kept in blood for many hours, CNPs only released small amounts of the drug; however, when exposed to light or agents such as glutathione, they readily released their payloads.
- The ability to precisely control chemotherapy release inside tumors could greatly reduce toxicity. CNPs carrying doxorubicin provided excellent cancer control in animals, with minimal side effects.
- CNPs can be configured to respond to light, producing singlet oxygen, reactive molecules that destroy tumor cells. They can also generate heat when hit with laser light. Significantly, CNPs can perform either task when exposed to a single wavelength of light.
- CNPs combine imaging and therapeutics, simultaneously delivering treatment and monitoring treatment efficacy. They readily chelate imaging agents and can remain in the body for long periods. In animal studies, CNPs congregated in tumors, making them easier to read on an MRI. Because CNPs accumulated in tumors, and not so much in normal tissue, they dramatically enhanced tumor contrast for MRI and may also be promising for PET-MRI scans.
- “These particles can also be used as optical probes for image-guided surgery,” said Kit Lam of the Department of Biochemistry and Molecular Medicine, UC Davis Comprehensive Cancer Center, University of California Davis. “In addition, they can be used as highly potent photosensitizing agents for intraoperative phototherapy.”
The Lam lab and its collaborators plan to pursue preclinical studies and, if all goes well, proceed to human trials.
This research was funded by the National Cancer Institute, National Institute of Biomedical Imaging and Bioengineering, the Department of Defense, the Prostate Cancer Foundation, the Veterans Administration, and the California Institute for Regenerative Medicine.
Abstract of Nature Communications paper
Multifunctional nanoparticles with combined diagnostic and therapeutic functions show great promise towards personalized nanomedicine. However, attaining consistently high performance of these functions in vivo in one single nanoconstruct remains extremely challenging. Here we demonstrate the use of one single polymer to develop a smart ‘all-in-one’ nanoporphyrin platform that conveniently integrates a broad range of clinically relevant functions. Nanoporphyrins can be used as amplifiable multimodality nanoprobes for near-infrared fluorescence imaging (NIRFI), magnetic resonance imaging (MRI), positron emission tomography (PET) and dual modal PET-MRI. Nanoporphyrins greatly increase the imaging sensitivity for tumour detection through background suppression in blood, as well as preferential accumulation and signal amplification in tumours. Nanoporphyrins also function as multiphase nanotransducers that can efficiently convert light to heat inside tumours for photothermal therapy (PTT), and light to singlet oxygen for photodynamic therapy (PDT). Furthermore, nanoporphyrins act as programmable releasing nanocarriers for targeted delivery of drugs or therapeutic radio-metals into tumours.