A ‘nanosubmarine’ that could deliver drug molecules to cells
July 31, 2014
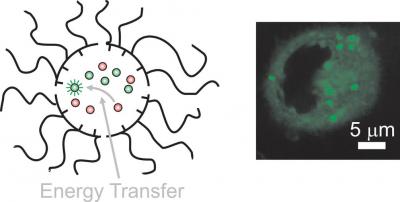
Nanocarriers transport donor and acceptor molecules across cell membranes with fluorescence activation (credit: Francisco Raymo)
Researchers at the University of Miami and the University of Ulster have created self-assembling nanoparticles that can transport drugs and other molecules into target living cells.
The new nanocarriers are just 15 nanometers in diameter, based on building blocks called amphiphilic polymers: they have both hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties). That allows the nanocarriers to hold the guest molecules within their water-insoluble interior and use their water-soluble exterior to travel through an aqueous environment. And that makes the nanocarriers ideal for transferring molecules that would otherwise be insoluble in water.
They also emit a fluorescent signal that can be observed with a microscope, allowing for tracking and photographing the nanoparticles in the body.
University of Miami | Francisco Raymo on nanocarrier research
“The size of these nanoparticles, their dynamic character and the fact that the reactions take place under normal biological conditions (at ambient temperature and neutral environment) makes these nanoparticles an ideal vehicle for the controlled activation of therapeutics, directly inside the cells,” says lead investigator Francisco Raymo, professor of chemistry in the University of Miami College of Arts and Sciences and UM laboratory for molecular photonics.
The next phase of this investigation involves demonstrating that this method can be used to achieve chemical reactions inside cells, instead of energy transfers.
This strategy is unique because it involves “sequential transport of interacting species inside cells,” Raymo explained in an email to KurzweilAI. “However, we are still very far from any commercial application at this stage.”
The study was published in the Journal of the American Chemical Society. Researchers at the University of Ulster were also involved in the research.
Abstract of Journal of the American Chemical Society paper
Decyl and oligo(ethylene glycol) chains were appended to the same poly(methacrylate) backbone to generate an amphiphilic polymer with a ratio between hydrophobic and hydrophilic segments of 2.5. At concentrations greater than 10 μg mL–1 in neutral buffer, multiple copies of this particular macromolecule assemble into nanoparticles with a hydrodynamic diameter of 15 nm. In the process of assembling, these nanoparticles can capture anthracene donors and borondipyrromethene acceptors within their hydrophobic interior and permit the transfer of excitation energy with an efficiency of 95%. Energy transfer is observed also if nanocarriers containing exclusively the donors are mixed with nanoparticles preloaded separately with the acceptors in aqueous media. The two sets of supramolecular assemblies exchange their guests with fast kinetics upon mixing to co-localize complementary chromophores within the same nanostructured container and enable energy transfer. After guest exchange, the nanoparticles can cross the membrane of cervical cancer cells and bring the co-entrapped donors and acceptors within the intracellular environment. Alternatively, intracellular energy transfer is also established after sequential cell incubation with nanoparticles containing the donors first and then with nanocarriers preloaded with the acceptors or vice versa. Under these conditions, the nanoparticles exchange their cargo only after internalization and allow energy transfer exclusively within the cell interior. Thus, the dynamic character of such supramolecular containers offers the opportunity to transport independently complementary species inside cells and permit their interaction only within the intracellular space.