A new dimension in breast cancer research
January 9, 2012
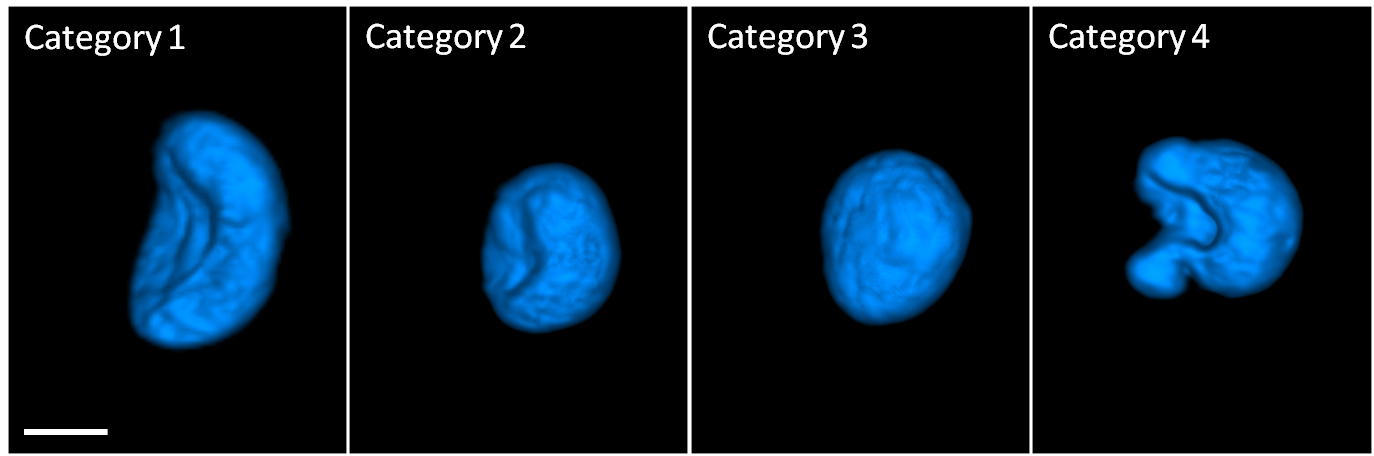
These surface-shaded renderings illustrate four nuclear shape categories of human mammary epithelial cells (scale bar = 5 microns) (credit: The Biodesign Institute at Arizona State University)
A new imaging technology under investigation at the Biodesign Institute at Arizona State University may help researchers pinpoint subtle aberrations in cell nuclear structure, the molecular biosignature of cancer, thus significantly improving diagnostic accuracy and prognosis by providing early detection of breast cancer, a leading worldwide health concern.
The team has examined normal, benign and malignant cells, using the Cell-CT from VisionGate, Inc., Phoenix, AZ, a specialized instrument capable of imaging cells in vivid 3D with true isotropic (all directions) resolution. The technology permits the examination of subtle cellular details inaccessible by more conventional forms of microscopy, which are inherently 2D.
The 3D movie images of cells observed in the study reveal numerous telltale traces of their condition as normal or aberrant. There are numerous quantitative morphological parameters that are indicative of disease and may be used as biosignatures for disease staging and diagnosis, says Professor Deirdre Meldrum, ASU Senior Scientist and Director of the Center for Biosignatures Discovery Automation at Biodesign. “For example, a cancerous cell typically has an enlarged nucleus, nuclear invaginations, chromosome mutations, and unique nuclear shape changes.”
Breast cancer remains the most common cancer in women. In 2011, an estimated 232, 000 new cases were diagnosed and some 39,000 fatalities occurred. Over a normal lifetime, 1 in 8 women will be diagnosed with the disease.
Currently, the definitive clinical diagnosis of malignancy relies on careful examination of the nuclear structure of cells that have been prepared by histological staining and subjected to bright field microscopy. According to Vivek Nandakumar, lead author of the current study, pathologists qualitatively examine cell features including nuclear size, shape, nucleus-to-cytoplasm ratio, and the texture of cell chromatin. However, these observations do not involve quantitative measurements that would promote a more accurate analysis.
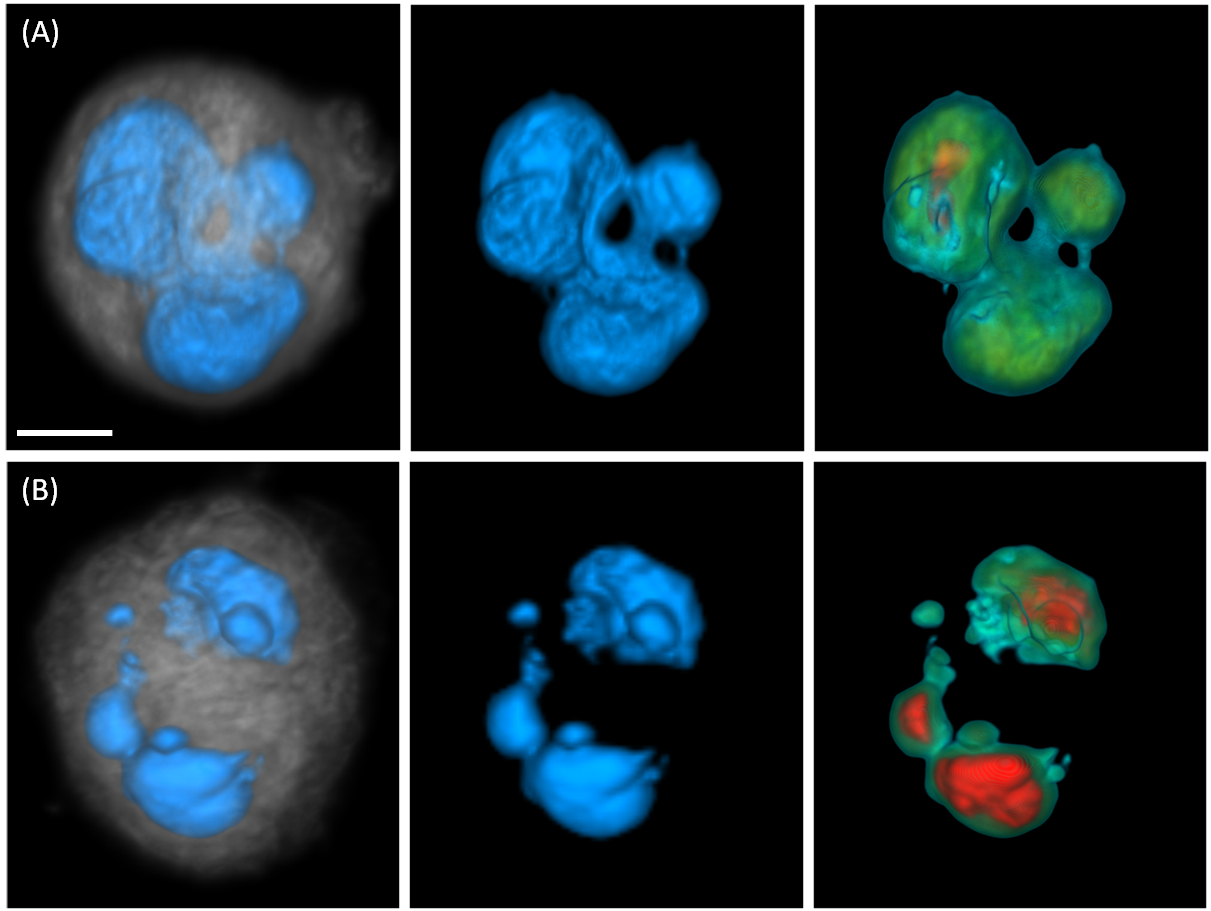
This image shows instances of irregular MDA-MB-231 nuclear morphologies (scale bar = 5 microns). Panel (A) depicts a multilobular nucleus and panel (B) illustrates a cell with micronuclei. Left images show nuclear surface in blue and cytoplasm in gray, middle images show surface-shaded renderings of the nuclear volume, and right images depict volume renderings through the nuclear volume. Increasing nuclear density is color coded from green to red. (Credit: The Biodesign Institute at Arizona State University)
The group used Cell-CT to examine 150 cells in each of three specific categories: normal, benign fibrocystic and malignant breast epithelial. Controversy remains as to whether breast fibrosis, which may result from hormonal changes, is a normal condition or an early harbinger of malignancy. The condition occurs when ligaments, scars, supportive tissue or other fibrous tissue become more prominent in the breast than fatty tissue.
Cell-CT is a new kind of microscope, able to image cells in three dimensions, using a technique called optical projection tomography. Cell-CT operates much like a normal CT scanner, though it uses visible photons of light, rather than X-rays. Cells prepared for observation are not placed on slides, but are instead suspended in gel and injected through a micro-capillary tube that permits multiple imaging in 360 degrees.
The scanning process produces hundreds of thin slices through the cell. These sections, or tomographs, are reassembled through computer software, forming a detailed 3-D portrait. Movies of cells seen in rotation brilliantly reveal shape asymmetries, a particularly useful tool for disease diagnosis.
Paul Davies, another co-author of the current study is a physicist and cosmologist in ASU’s College of Liberal Arts and Sciences and part of a new National Cancer Institute funded consortium, devoted to studying the physical science of cancer: “We expect that insights and methods drawn from physical science will lead to radical new ideas for understanding and tackling cancer,” says Davies.
The group’s results provide a new window on the variations of nuclear structure that often signal cell malignancy. The unparalleled structural details produced by Cell-CT promise to dramatically improve 3-D nuclear morphometry, leading to a sensitive and specific nuclear grade classification for breast cancer diagnosis, the researchers believe.
Ref.: Vivek Nandakumar, Isotropic 3D Nuclear Morphometry of Normal, Fibrocystic and Malignant Breast Epithelial Cells Reveals New Structural Alterations, PLoS ONE, 2012; [DOI:10.1371/journal.pone.0029230]