A new tool for precise brain mapping
May 21, 2013
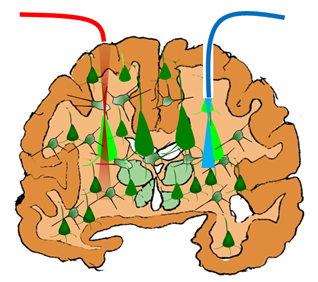
Non-invasive, neuron-specific localized stimulation by near-IR fiber optic beam (red) vs. invasive, non-localized stimulation by blue light (credit: S. Mohanty/UT Arlington)
A new tool that could help map and track the interactions between neurons in different areas of the brain is being developed by University of Texas Arlington assistant professor of physics Samarendra Mohanty.
The technology would be useful in the BRAIN (Brain Research Through Advancing Innovative Neurotechnologies) mapping initiative.
This new method, which uses a fiber-optic, two-photon, optogenetic stimulator, has been used on human cells in a laboratory, but is also expected to work in vivo. Optogenetic stimulation avoids damage to living tissue by using light to stimulate neurons instead of the electric pulses used in past research.
“Scientists have spent a lot of time looking at the physical connections between different regions of the brain. But that information is not sufficient unless we examine how those connections function,” Mohanty said. “That’s where two-photon optogenetics comes into play. This is a tool not only to control the neuronal activity but to understand how the brain works.”
How it works
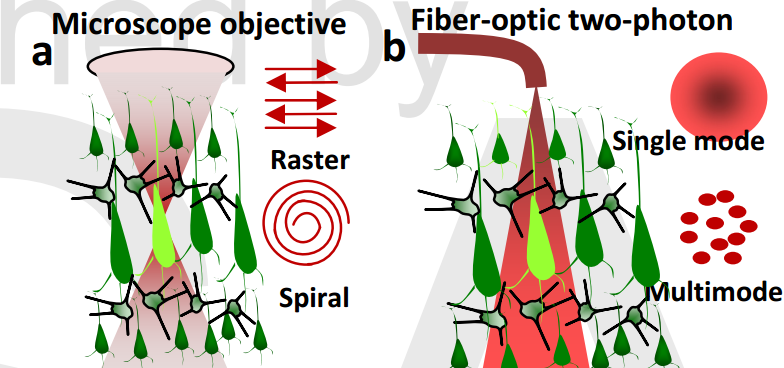
(a) Schematic of conventional two-photon stimulation scanning pattern of targeted cell with laser beam delivered by microscope. (b) Schematic of fiber-optic two-photon activation. (Credit: S. Mohanty et al./Optics Letters)
The tiny tool builds on Mohanty’s previous discovery that near-infrared light can be used to stimulate an opsin (a light-sensitive protein) introduced into neurons in the brain. Most opsins are currently activated in the visible spectrum, where significant absorption and scattering of stimulating light occurs, leading to low penetration depth. This new method could also show how different parts of the brain react when a linked area is stimulated, Mohanty said.
The two-photon optogenetic stimulation involves introducing the gene for an opsin called ChR2 into a sample of excitable cells (neurons in this case). A fiber-optic infrared beam of light can then be used to precisely excite the neurons in a tissue circuit. Researchers can then observe responses in the excited area as well as other parts of the neural circuit. In living subjects, scientists could also observe the behavioral outcome, Mohanty said.
Mohanty’s method of using low-energy near-infrared light (which penetrates tissue better) also enables more precision and a deeper penetration than the blue or green light beams often used in optogenetic stimulation, according to the Optics Letters paper.
Using fiber optics to deliver the two-photon optogenetic beam is another advance. Previous methods required bulky microscopes or complex scanning beams.
Mohanty’s group is collaborating with UT Arlington Department of Psychology assistant professor Linda Perrotti to apply this technology in living animals.