A self-assembling molecular nanoswitch
January 18, 2016
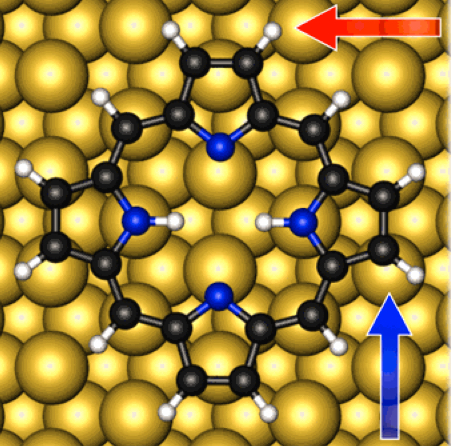
Molecular nanoswitch: calculated adsorption geometry of porphine adsorbed at copper bridge site (credit: Moritz Müller et al./J. Chem. Phys.)
Technical University of Munich (TUM) researchers have simulated a self-assembling molecular nanoswitch in a supercomputer study.
As with other current research in bottom-up self-assembly nanoscale techniques, the goal is to further miniaturize electronic devices, overcoming the physical limits of currently used top-down procedures such as photolithography.
The new TUM research focuses on porphine (C20H14N4, the simplest form of porphyrin* organic molecules), interacting on copper and silver surfaces to form a single-porphyrin switch that occupies a surface area of only one square nanometer (porphine itself is much smaller). Porphyrins have potential applications in molecular memory devices, photovoltaics, gas sensors, light emission, and catalysis, the researchers note.
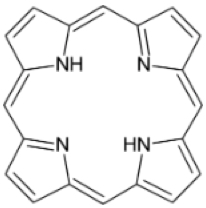
Structure of a porphine molecule (credit: Moritz Müller et al./J. Chem. Phys.)
In their simulation, the researchers placed porphine molecules on a copper or silver slab. After finding the optimal geometry in which the molecules would adsorb on the surface, the researchers altered the size of the metal slab to increase or decrease the distance between molecules to simulate different molecular coverages.
The researchers found that weak long-range van der Waals (attractive or repulsive forces between molecules or atomic groups that do not arise from interactions due to a covalent bond or electrostatic force) yielded the largest contribution to the molecule-surface interaction.
The study was published last week in The Journal of Chemical Physics.
* Porphyrins are a group of ringed chemical compounds which notably include heme — responsible for transporting oxygen and carbon dioxide in the bloodstream — and chlorophyll. Porphyrins are studied in the lab for their potential uses as sensors, light-sensitive dyes in organic solar cells, and molecular magnets. The close-packed single crystal surfaces of copper and silver, are widely used as substrates in surface science. This is due to the densely packed nature of the surfaces, which allow the molecules to exhibit a smooth adsorption environment. Additionally, copper and silver each react differently with porhyrins. These molecules adsorb more strongly on copper, whereas silver does a better job of keeping the electronic structure of the molecule intact — allowing the researchers to monitor a variety of competing effects for future applications.
Abstract of Interfacial charge rearrangement and intermolecular interactions: Density-functional theory study of free-base porphine adsorbed on Ag(111) and Cu(111)
We employ dispersion-corrected density-functional theory to study the adsorption of tetrapyrrole 2H-porphine (2H-P) at Cu(111) and Ag(111). Various contributions to adsorbate-substrate and adsorbate-adsorbate interactions are systematically extracted to analyze the self-assembly behavior of this basic building block to porphyrin-based metal-organic nanostructures. This analysis reveals a surprising importance of substrate-mediated van der Waals interactions between 2H-P molecules, in contrast to negligible direct dispersive interactions. The resulting net repulsive interactions rationalize the experimentally observed tendency for single molecule adsorption.