‘Artificial leaf’ harnesses sunlight for efficient, safe hydrogen fuel production
August 28, 2015
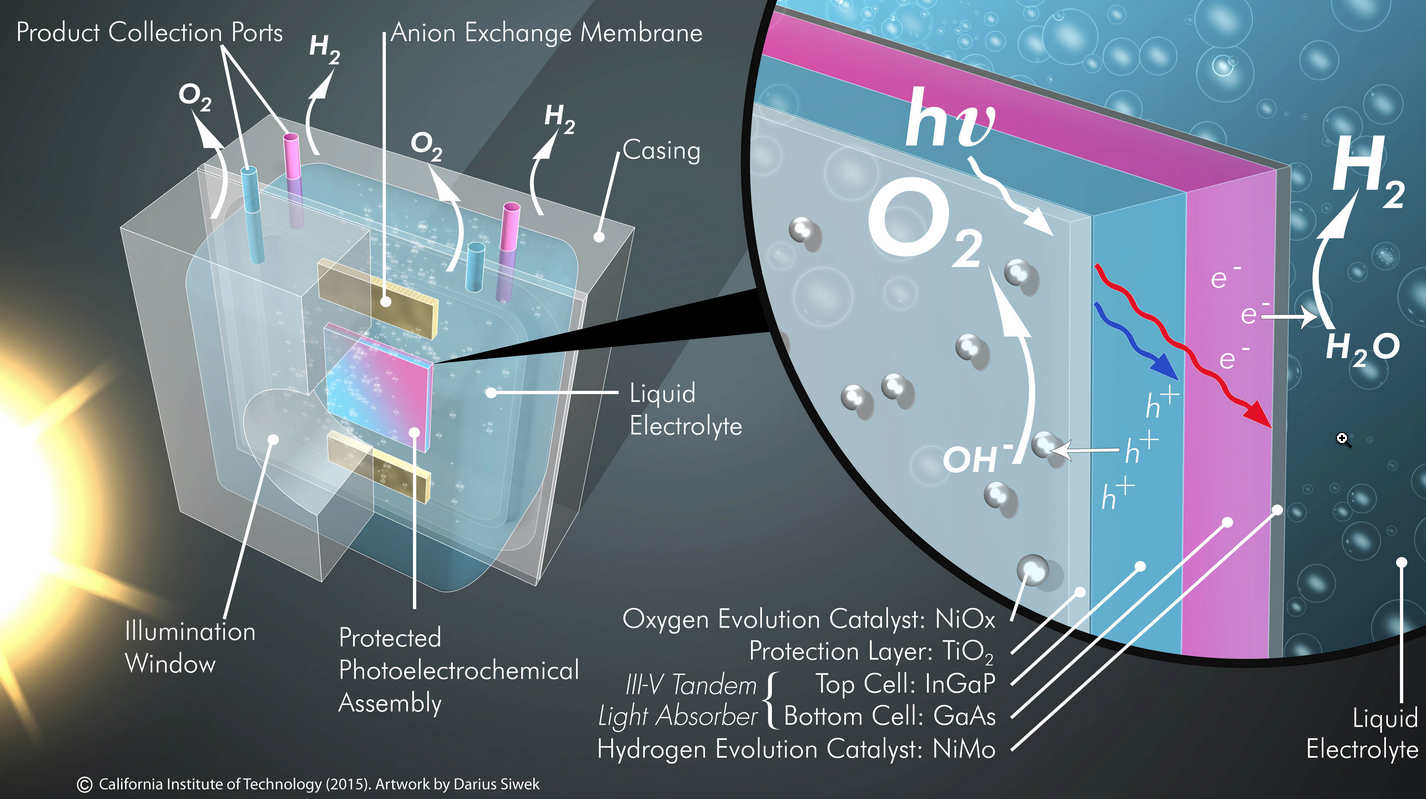
Illustration of an efficient, robust and integrated solar-driven prototype featuring protected photoelectrochemical assembly coupled with oxygen and hydrogen evolution reaction catalysts (credit: Image, Joint Center for Artificial Photosynthesis; artwork, Darius Siwek)
The first complete, efficient, safe, integrated solar-driven system for splitting water to create hydrogen fuels has been developed by the Joint Center for Artificial Photosynthesis (JCAP) at Caltech, according to Caltech’s Nate Lewis, George L. Argyros Professor and professor of chemistry, and the JCAP scientific director.
The new solar fuel generation system, or “artificial leaf,” is described in the August 27 online issue of the journal Energy and Environmental Science. The work was done by researchers in the laboratories of Lewis and Harry Atwater, director of JCAP and Howard Hughes Professor of Applied Physics and Materials Science.

A highly efficient photoelectrochemical (PEC) device uses the power of the sun to split water into hydrogen and oxygen. The stand-alone prototype includes two chambers separated by a semi-permeable membrane that allows collection of both gas products. (credit: Lance Hayashida/Caltech)
The new system consists of three main components: two electrodes (photoanode and photocathode) and a membrane.
- The photoanode uses sunlight to oxidize water molecules, generating protons and electrons as well as oxygen gas.
- The photocathode recombines the protons and electrons to form hydrogen gas.
- A plastic membrane keeps the oxygen and hydrogen gases separate. (If the two gases are allowed to mix and are accidentally ignited, an explosion can occur; the membrane lets the hydrogen fuel be separately collected under pressure and safely pushed into a pipeline.)
Preventing corrosion
Semiconductors such as silicon or gallium arsenide absorb light efficiently and are therefore used in solar panels. However, these materials also oxidize (or rust) on the surface when exposed to water, so cannot be used to directly generate fuel. A major advance that allowed the integrated system to be developed was previous work in Lewis’s laboratory, which showed that adding a nanometers-thick layer of titanium dioxide (TiO2) onto the electrodes could prevent them from corroding while still allowing light and electrons to pass through.
The new complete solar fuel generation system developed by Lewis and colleagues uses such a 62.5-nanometer-thick TiO2 layer to effectively prevent corrosion and improve the stability of a gallium arsenide–based photoelectrode.
Inexpensive catalysts
Another key advance is the use of active, inexpensive catalysts for fuel production. The photoanode requires a catalyst to drive the essential water-splitting reaction. Rare and expensive metals such as platinum can serve as effective catalysts, but in its work the team discovered that it could create a much cheaper, active catalyst by adding a 2-nanometer-thick layer of nickel to the surface of the TiO2. This catalyst is among the most active known catalysts for splitting water molecules into oxygen, protons, and electrons and is a key to the high efficiency displayed by the device.
The photoanode was grown onto a photocathode, which also contains a highly active, inexpensive, nickel-molybdenum catalyst, to create a fully integrated single material that serves as a complete solar-driven water-splitting system.
The demonstration system is approximately one square centimeter in area, converts 10 percent of the energy in sunlight into stored energy in the chemical fuel, and can operate for more than 40 hours continuously.
“This new system shatters all of the combined safety, performance, and stability records for artificial leaf technology by factors of 5 to 10 or more,” Lewis says.
“Our work shows that it is indeed possible to produce fuels from sunlight safely and efficiently in an integrated system with inexpensive components,” Lewis adds, “Of course, we still have work to do to extend the lifetime of the system and to develop methods for cost-effectively manufacturing full systems, both of which are in progress.”
Caltech | Solar Fuels Prototype in Operation
Abstract of A monolithically integrated, intrinsically safe, 10% efficient, solar-driven water-splitting system based on active, stable earth-abundant electrocatalysts in conjunction with tandem III–V light absorbers protected by amorphous TiO2 films
A monolithically integrated device consisting of a tandem-junction GaAs/InGaP photoanode coated by an amorphous TiO2 stabilization layer, in conjunction with Ni-based, earth-abundant active electrocatalysts for the hydrogen-evolution and oxygen-evolution reactions, was used to effect unassisted, solar-driven water splitting in 1.0 M KOH(aq). When connected to a Ni–Mo-coated counterelectrode in a two-electrode cell configuration, the TiO2-protected III–V tandem device exhibited a solar-to-hydrogen conversion efficiency, ηSTH, of 10.5% under 1 sun illumination, with stable performance for >40 h of continuous operation at an efficiency of ηSTH > 10%. The protected tandem device also formed the basis for a monolithically integrated, intrinsically safe solar-hydrogen prototype system (1 cm2) driven by a NiMo/GaAs/InGaP/TiO2/Ni structure. The intrinsically safe system exhibited a hydrogen production rate of 0.81 μL s−1 and a solar-to-hydrogen conversion efficiency of 8.6% under 1 sun illumination in 1.0 M KOH(aq), with minimal product gas crossover while allowing for beneficial collection of separate streams of H2(g) and O2(g).