digest | at First Sight: artificial retina for patients
April 1, 2019
note: This post is being updated and is under construction for May 12. Thanks for your patience!
IMAGE
— contents —
~ story
~
~ reading
— the story —
The Food + Drug Administration of the United States granted market approval to an artificial retina technology, the first bionic eye to be approved for patients.
VIDEO
www.nsf.gov/news/news_images.jsp?cntn_id=126756
sectional TRANSCRIBE
https://www.google.com/search?client=firefox-b-1-d&q=argus+11
https://www.google.com/search?client=firefox-b-1-d&q=argus+11
—————————————–

Argus II (credit: Second Sight)
The device, called the Argus II Retinal Prosthesis System, from Second Sight Medical Products co., transmits images from a small, eye-glass-mounted camera wirelessly to a microelectrode array implanted on a patient’s damaged retina. The array sends electrical signals via the optic nerve, and the brain interprets a visual image.
The FDA approval currently applies to individuals who have lost sight as a result of severe to profound retinitis pigmentosa (RP), an ailment that affects one in every 4,000 Americans. The implant allows some individuals with RP, who are completely blind, to locate objects, detect movement, improve orientation and mobility skills and discern shapes such as large letters.
How it works
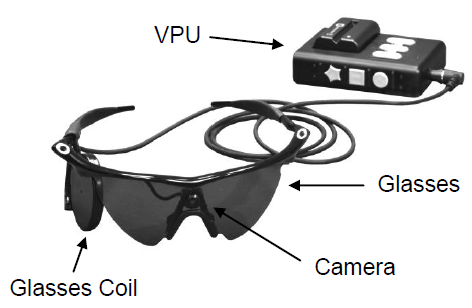
Argus II components (credit: FDA)
The Argus II design consists of an external video camera system matched to the implanted retinal stimulator, which contains a microelectrode array that spans 20 degrees of visual field.
An external camera system, built into a pair of glasses, streams video to a belt-worn computer, which converts the video into stimulus commands for the implant.
The belt-worn video processing unit (computer) encodes the commands into a wireless signal that is transmitted to the implant, which has the necessary electronics to receive and decode both wireless power and data.
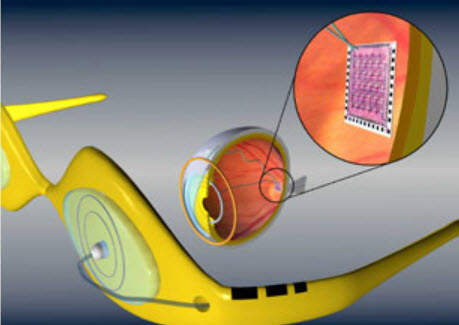
Artificial retina device, consisting of a glasses-mounted camera and a microchip surgically implanted on the retina (credit: Dr. Wentai Liu)
Based on those data, the implant stimulates the retina with small electrical pulses. The electronics are hermetically packaged and the electrical stimulus is delivered to the retina via a microelectrode array.
In 1998, Robert Greenberg founded Second Sight to develop the technology for the marketplace. While under development, the Argus I and Argus II systems have won wide recognition.
The prosthetic technology has received early and continuing support from the National Science Foundation (NSF), the National Institutes of Health and the Department of Energy, with grants totaling more than $100 million. The private sector’s support nearly matched that of the federal government.
The NSF BMES ERC also developed a prototype system with an array of more than 15 times as many electrodes and an ultra-miniature video camera that can be implanted in the eye. However, this prototype is many years away from being available for patient use.
on the web | reading
NSF | Artificial Retina: may restore partial sight for 1,000s in the next decade
deck: Research by engineering + medical teams holds tremendous promise.
deck: For people who lost their sight from retinitis pigmentosa + degenerative eye diseases.
NSF | Artificial Retina: receives FDA approval
deck: Prosthesis to restore limited vision to those blinded by retinitis pigmentosa.
bionic eye
Blind patient reads words stimulated directly onto the retina
Blind patient reads words stimulated directly onto the retina
— notes —
NSF = National Science Foundation of the United States
FDA = Food + Drug Admin. of the United States