Automated drug design using synthetic DNA self-assembly
December 6, 2012

Pharmaceutical molecules after self-assembly. The detail shows a single molecule, made up of strands of DNA (credit: Parabon NanoLabs)
Using a simple “drag-and-drop” computer interface and DNA self-assembly techniques, Parabon NanoLabs researchers have developed a new automated method of drug development that could reduce the time required to create and test medications, with the support of an NSF Technology Enhancement for Commercial Partnerships grant.
“We can now ‘print,’ molecule by molecule, exactly the compound that we want,” says Steven Armentrout, the principal investigator on the NSF grants and co-developer of Parabon’s technology.
“What differentiates our nanotechnology from others is our ability to rapidly, and precisely, specify the placement of every atom in a compound that we design.”
The Parabon Essemblix Drug Development Platform combines computer-aided design (CAD) software with nanoscale fabrication technology, developed in partnership with Janssen Research & Development, LLC, part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
To develop new drugs, scientists can use the CAD software to design molecular pieces with specific, functional components. The software then optimizes the design using a cloud supercomputing platform that uses proprietary algorithms to search for specific sets of DNA sequences that can self-assemble those components.
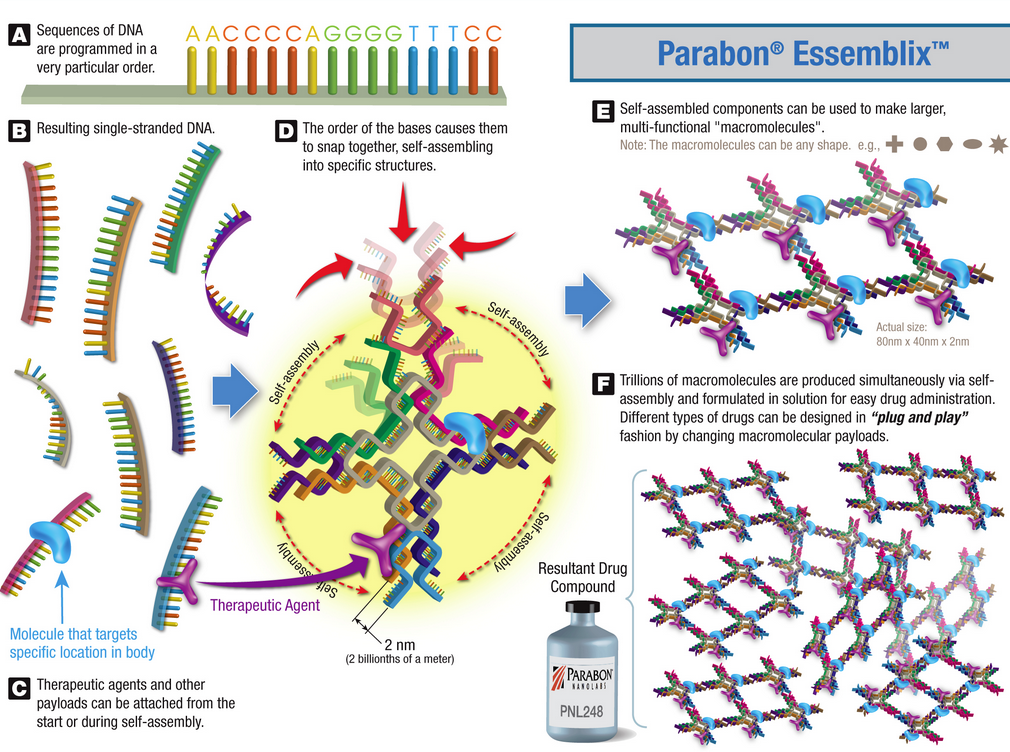
Parabon Essemblix process (credit: Parabon NanoLabs)
“When designing a therapeutic compound, we combine knowledge of the cell receptors we are targeting or biological pathways we are trying to affect with an understanding of the linking chemistry that defines what is possible to assemble,” says Hong Zhong, senior research scientist at Parabon and a collaborator on the grants. “It’s a deliberate and methodical engineering process, which is quite different from most other drug development approaches in use today.”
With the resulting sequences, the scientists chemically synthesize trillions of identical copies of the designed molecules. The process, from conception to production, can be performed in weeks, or even days — much faster than traditional drug discovery techniques that rely on trial and error for screening potentially useful compounds, the company says.
In vivo experiments, funded by the NSF SBIR award, validated the approach, and Parabon filed a provisional patent for its methods and compounds on May 4, 2011. The final application was published in 2012.
Faster, more targeted rational drug design
The process is characteristic of rational drug design, an effort to craft pharmaceuticals based on knowledge of how certain molecular pieces will work together in a biological system. For example, some molecules are good at finding cancer cells, while others are good at latching on to cancer cells, while still others are capable of killing cells. Working together as part of a larger molecule, these pieces could prove effective as a cancer treatment.
While there are other methods to create multi-component compounds, they generally take more time, and the majority of them lack the precise control over size, charge and the relative placement of components enabled by the new technology, the company says.
The Parabon and Janssen researchers intend for their new prostate cancer drug to overcome several existing cancer-treatment obstacles. The drug design combines a toxin with a chemical that makes cancer cells susceptible to that toxin. The drug also incorporates components that improve delivery to cancer cells while avoiding healthy tissue, and chemical markers that allow researchers to monitor the drug’s arrival at tumors. For the new compound, total design time plus synthesis time will be a matter of weeks.
“Currently, most drugs are developed using a screening technique where you try a lot of candidate compounds against targets to ‘see what sticks’,” says Armentrout. “Instead, we’re designing very specific drugs based on their molecular structure, with target molecules that bind to receptors on specific types of cancer cells. In plug-and-play fashion, we can swap in or swap out any of the functional components, as needed, for a range of treatment approaches.”
Concurrently, Parabon is developing other applications for the technology, including synthetic vaccines for biodefense and gene therapies that can target disease, based on information from an individual’s genome. The technology also has applications outside of medicine, and Parabon’s co-founders Chris Dwyer and Michael Norton are building upon the initial NSF-supported work to develop processes to create nanoscale logic gates, devices critical for computing, and molecular nanosensors.