Best textile manufacturing methods for creating human tissues with stem cells
April 8, 2016
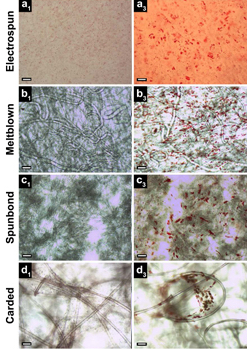
All four textile manufacturing processes and corresponding scaffold (structure) types studied exhibited the presence of lipid vacuoles (small red spheres, right column, indicating stem cells undergoing random differentiation), compared to control (left). Electrospun scaffolds (row a) exhibited only a monolayer of lipid vacuoles in a single focal plane, while meltblown, spunbond, and carded scaffolds (rows b, c, d) exhibited vacuoles in multiple planes throughout the fabric thickness. Scale bars: 100 μm (credit: S. A. Tuin et al./Biomedical Materials)
Elizabeth Loboa, dean of the Missouri University College of Engineering, and her team have tested new tissue- engineering methods (based on textile manufacturing) to find ones that are most cost-effective and can be produced in larger quantities.
Tissue engineering is a process that uses novel biomaterials seeded with stem cells to grow and replace missing tissues. When certain types of materials are used, the “scaffolds” that are created to hold stem cells eventually degrade, leaving natural tissue in its place. The new tissues could help patients suffering from wounds caused by diabetes and circulation disorders, patients in need of cartilage or bone repair, and women who have had mastectomies by replacing their breast tissue. The challenge is creating enough of the material on a scale that clinicians need to treat patients.
Comparing textile manufacturing techniques
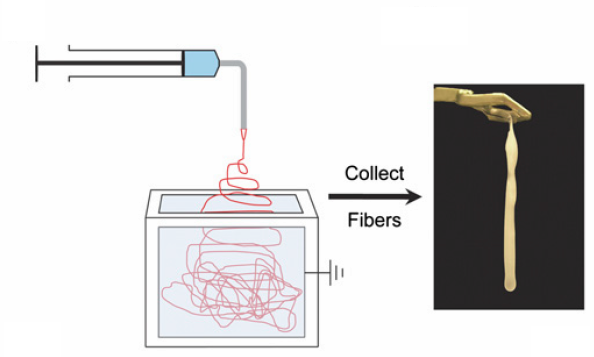
Electrospinning experiment: nanofibers are collected into an ethanol bath and removed at predefined time intervals (credit: J. M. Coburn et al./The Johns Hopkins University/PNAS)
In typical tissue engineering approaches that use fibers as scaffolds, non-woven materials are often bonded together using an electrostatic field. This process, called electrospinning (see Nanoscale scaffolds and stem cells show promise in cartilage repair and Improved artificial blood vessels), creates the scaffolds needed to attach to stem cells.
However, large-scale production with electrospinning is not cost-effective. “Electrospinning produces weak fibers, scaffolds that are not consistent, and pores that are too small,” Loboa said. “The goal of ‘scaling up’ is to produce hundreds of meters of material that look the same, have the same properties, and can be used in clinical settings. So we investigated the processes that create textiles, such as clothing and window furnishings like drapery, to scale up the manufacturing process.”
The group published two papers using three industry-standard, high-throughput manufacturing techniques — meltblowing, spunbonding, and carding — to determine if they would create the materials needed to mimic native tissue.
Meltblowing is a technique during which nonwoven materials are created using a molten polymer to create continuous fibers. Spunbond materials are made much the same way but the fibers are drawn into a web while in a solid state instead of a molten one. Carding involves the separation of fibers through the use of rollers, forming the web needed to hold stem cells in place.
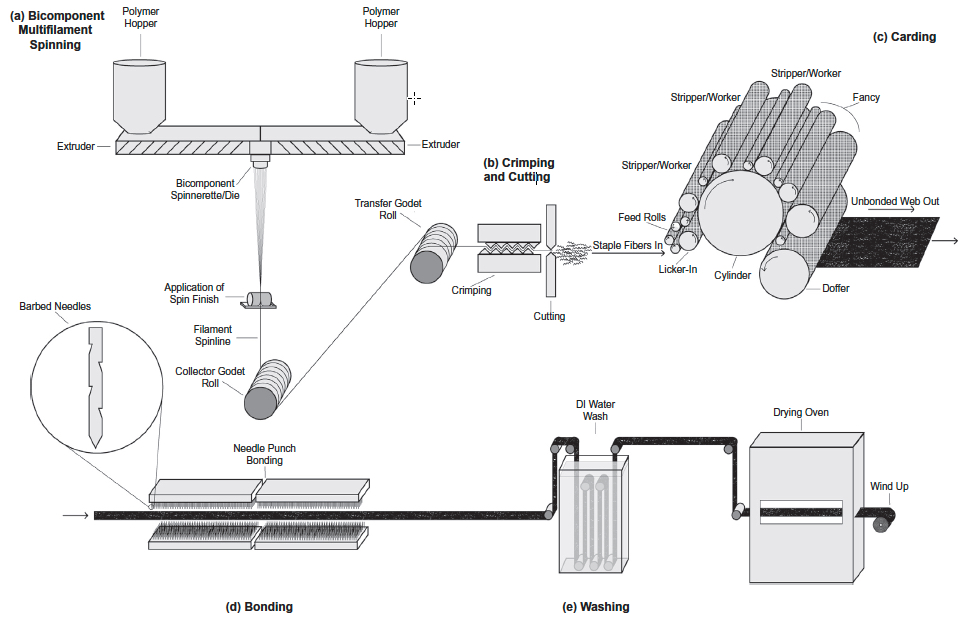
Schematic of gilled fiber multifilament spinning and carded scaffold fabrication (credit: Stephen A. Tuin et al./Acta Biomaterialia)
Cost-effective methods
Loboa and her colleagues tested these techniques to create polylactic acid (PLA) scaffolds (a Food and Drug Administration-approved material used as collagen fillers), seeded with human stem cells. They then spent three weeks studying whether the stem cells remained healthy and if they began to differentiate into fat and bone pathways, which is the goal of using stem cells in a clinical setting when new bone and/or new fat tissue is needed at a defect site. Results showed that the three textile manufacturing methods proved as viable if not more so than electrospinning.
“These alternative methods are more cost-effective than electrospinning,” Loboa said. “A small sample of electrospun material could cost between $2 to $5. The cost for the three manufacturing methods is between $.30 to $3.00; these methods proved to be effective and efficient. Next steps include testing how the different scaffolds created in the three methods perform once implanted in animals.”
Researchers at North Carolina State University and the University of North Carolina at Chapel Hill were also involved in the two studies, which were published in Biomedical Materials (open access) and Acta Biomaterialia. The National Science Foundation, the National Institutes of Health, and the Nonwovens Institute provided funding for the studies.
Abstract of Creating tissues from textiles: scalable nonwoven manufacturing techniques for fabrication of tissue engineering scaffolds
Electrospun nonwovens have been used extensively for tissue engineering applications due to their inherent similarities with respect to fibre size and morphology to that of native extracellular matrix (ECM). However, fabrication of large scaffold constructs is time consuming, may require harsh organic solvents, and often results in mechanical properties inferior to the tissue being treated. In order to translate nonwoven based tissue engineering scaffold strategies to clinical use, a high throughput, repeatable, scalable, and economic manufacturing process is needed. We suggest that nonwoven industry standard high throughput manufacturing techniques (meltblowing, spunbond, and carding) can meet this need. In this study, meltblown, spunbond and carded poly(lactic acid) (PLA) nonwovens were evaluated as tissue engineering scaffolds using human adipose derived stem cells (hASC) and compared to electrospun nonwovens. Scaffolds were seeded with hASC and viability, proliferation, and differentiation were evaluated over the course of 3 weeks. We found that nonwovens manufactured via these industry standard, commercially relevant manufacturing techniques were capable of supporting hASC attachment, proliferation, and both adipogenic and osteogenic differentiation of hASC, making them promising candidates for commercialization and translation of nonwoven scaffold based tissue engineering strategies.
Abstract of Fabrication of novel high surface area mushroom gilled fibers and their effects on human adipose derived stem cells under pulsatile fluid flow for tissue engineering applications
The fabrication and characterization of novel high surface area hollow gilled fiber tissue engineering scaffolds via industrially relevant, scalable, repeatable, high speed, and economical nonwoven carding technology is described. Scaffolds were validated as tissue engineering scaffolds using human adipose derived stem cells (hASC) exposed to pulsatile fluid flow (PFF). The effects of fiber morphology on the proliferation and viability of hASC, as well as effects of varied magnitudes of shear stress applied via PFF on the expression of the early osteogenic gene marker runt related transcription factor 2 (RUNX2) were evaluated. Gilled fiber scaffolds led to a significant increase in proliferation of hASC after seven days in static culture, and exhibited fewer dead cells compared to pure PLA round fiber controls. Further, hASC-seeded scaffolds exposed to 3 and 6 dyn/cm2 resulted in significantly increased mRNA expression of RUNX2 after one hour of PFF in the absence of soluble osteogenic induction factors. This is the first study to describe a method for the fabrication of high surface area gilled fibers and scaffolds. The scalable manufacturing process and potential fabrication across multiple nonwoven and woven platforms makes them promising candidates for a variety of applications that require high surface area fibrous materials.
Statement of Significance
We report here for the first time the successful fabrication of novel high surface area gilled fiber scaffolds for tissue engineering applications. Gilled fibers led to a significant increase in proliferation of human adipose derived stem cells after one week in culture, and a greater number of viable cells compared to round fiber controls. Further, in the absence of osteogenic induction factors, gilled fibers led to significantly increased mRNA expression of an early marker for osteogenesis after exposure to pulsatile fluid flow. This is the first study to describe gilled fiber fabrication and their potential for tissue engineering applications. The repeatable, industrially scalable, and versatile fabrication process makes them promising candidates for a variety of scaffold-based tissue engineering applications.