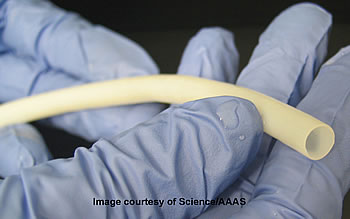
Bioengineered veins could help patients needing bypass surgery, dialysis
February 3, 2011
New research published in the current issue of the journal Science Translational Medicine demonstrates the capability of tissue-engineered vascular grafts that are immediately available at the time of surgery and are less likely to become infected or obstructed. A surgeon could pull a new human vein off the shelf for use in life-saving vascular surgeries.
The bioengineering method of producing veins shows promise in large- and small-diameter applications, such as for coronary artery bypass surgery and for vascular access in hemodialysis.
Humacyte, a Morrisville biotechnology company, worked with university researchers to develop the veins.
“This new type of bioengineered vein allows them to be easily stored in hospitals so they are readily available to surgeons at the time of need,” said Dr. Alan P. Kypson, a cardiothoracic surgeon, associate professor at the Brody School of Medicine at ECU and an author of the paper. “Currently, grafting using the patient’s own veins remains the gold standard. But, harvesting a vein from the patient’s leg can lead to complications, and for patients who don’t have suitable veins, the bioengineered veins could serve as an important new way to provide a coronary bypass.”
The American Heart Association Update on Heart Disease Statistics reports that in 2007, in the United States, surgeons performed more than 400,000 coronary bypass procedures. Patients requiring bypass surgery may not have suitable veins or arteries available and are not candidates for synthetic grafts because of the size needed for grafting.
The bioengineered veins also show promise for patients on kidney hemodialysis. According to the National Kidney Foundation, 320,000 patients are on chronic hemodialysis. Each year, 110,000 new patients develop renal failure requiring dialysis, and the number is growing by 3 percent a year. More than half of dialysis patients lack the healthy veins necessary and must undergo an arteriovenous graft placement to have bloodstream access for hemodialysis.
Most arteriovenous grafts that are placed for hemodialysis access are made of a synthetic material, which suffers from significant drawbacks including a high rate of infection, a propensity for blockages due to clotting and a thickening of blood vessels known as intimal hyperplasia, said Dr. Jeffrey H. Lawson, a surgeon and associate professor at Duke University School of Medicine and an author of the research.
“Due to high complication rates, each AV dialysis graft requires an average of 2.8 interventions over its lifetime just to keep it functioning,” Lawson said. “Hence, there is a huge clinical need for a functionally superior, off-the-shelf AV graft that suffers from fewer complications than current materials.”
Lawson has served as a consultant for Humacyte and has received research support from the company through Duke.
In this research, scientists generated bioengineered veins in a bioreactor — a device designed to support a biological environment — and then stored them up to 12 months in refrigerated conditions. The bioengineered veins, 3 millimeters to 6 millimeters in diameter, demonstrated excellent blood flow and resistance to blockage in large animal models for up to a year.
Scientists from Duke, ECU, Yale University and Humacyte conducted the research, and Humacyte, a leader in regenerative medicine, funded it. Overseeing the research and serving as senior author of the article was Dr. Laura Niklason, founder of Humacyte and professor of anesthesiology and biomedical engineering at Yale. Niklason is an authority in regenerative medicine for arterial engineering and led the team that recently created a functioning rat lung in a laboratory.
Shannon L.M. Dahl, senior director of scientific operations and co-founder of Humacyte, is lead author on the paper. “Not only are bioengineered veins available at the time of patient need, but the ability to generate a significant number of grafts from a cell bank will allow for a reduction in the final production costs, as compared to other regenerative medicine strategies,” Dahl said. “While there is still considerable research to be done before a product is available for widespread use, we are highly encouraged by the results outlined in this paper and eager to move forward with additional study.”
Humacyte, a privately held company, is primarily focused on developing products for vascular disease and for dermal filling and soft tissue repair. The company uses its innovative and proprietary platform technology to engineer human extracellular matrix-based tissues that can be shaped into tubes, sheets or particulate conformations with properties similar to native tissues.
These can then be used in many specific applications, with the potential to significantly improve treatment outcomes for a variety of patients, including those with diabetes and on hemodialysis. The company’s proprietary technologies are designed to result in off-the-shelf products that can be used in any patient.
Ref.: “Readily Available Tissue-Engineered Vascular Grafts,” Science Translational Medicine, Vol. 3, Issue 68, p. 68ra9
Adapted from materials provided by East Carolina University