Body maps, plasticity, and neurological disorders
July 25, 2013
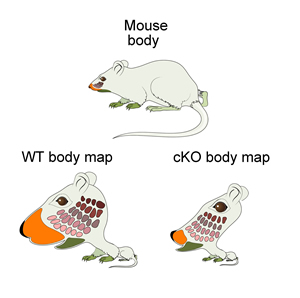
Sense receptors distributed over the body surface are represented in corresponding sensory maps in somatosensory cortex (S1).Their size in S1 is dictated by the number and density of sense receptors and nerves that connect each body part with S1. A normal “Mouseunculus” (WT) contains highly enlarged maps representing the face and whiskers. In animals lacking Pax6 in the neocortex (cKO), all body maps are very small and incomplete, resulting in a highly distorted “Mouseunculus” that shows abnormal somatosensation in these mice. (Credit: Andreas Zembrzycki and Jamie Simon, Salk Institute for Biological Studies)
Salk Institute researchers have demonstrated that altering the functional architecture of the brain’s cortex is possible, and that this alteration produces significant changes in parts of the brain that connect with the cortex and define its functional properties.
Dennis O’Leary, holder of the Vincent J. Coates Chair of Molecular Neurobiology at Salk, was the first scientist to show that the basic functional architecture of the cortex, the largest part of the human brain, was genetically determined during development.
But O’Leary wondered: what if the layout of the cortex wasn’t fixed? What would happen if it were changed?
Such changes may lay at the heart of neural developmental problems, such as autism spectrum disorders (ASD), O’Leary and his team suggest.
How sizes of cortex areas differ
The human cortex is involved in higher functions such as sensory perception, spatial reasoning, conscious thought and language. All mammals have areas in the cortex that process the senses, but they have them in different proportions.
Mice are nocturnal, so they have a large somatosensory area (S1) in the cortex, responsible for somatosensation, or feelings of the body that include touch, pain, temperature and proprioception.
“The area layout of the cortex directly relates to the lifestyle of an animal,” says Zembrzycki. “Areas are bigger or smaller according to the functional needs of the animal, not the physical size of the body parts from which they receive input.”
Even with relative sizes to other species set in place, areas in the cortex of humans may differ greatly across individuals. Such variations may underlie why some people appear to be naturally better at certain perceptual tasks, such as hitting a baseball or detecting the details of visual illusions. In patients with neurological disorders, there is an even wider range of differences.
Body maps
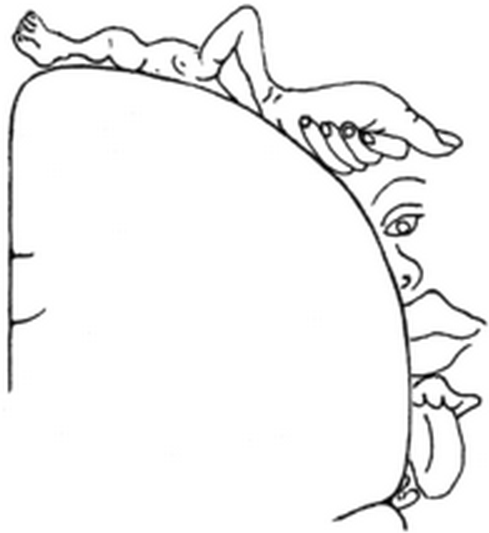
The cortical sensory homunculus (credit: Wilder Penfield)
The neurons in S1 are arranged in functional groups called “body maps” according to the density of nerve endings in the skin; thus, there’s a larger group of neurons dedicated to the skin on the face, than the skin on the legs.
Neurosurgeon Wilder Penfield famously illustrated this idea as a “sensory homunculus,” a cartoon of disproportionately sized body parts arching over the cortex.
Mice have a similar “mouseunculus” in their cortex in which the body map of the facial whiskers is highly enlarged.
These perceptual maps are not set for life. For example, if innervation of a body part is diminished early in life during a critical period, its map may shrink, while other parts of the body map may grow in compensation.
This is a version of “bottom-up plasticity,” in which external experience affects body maps in the brain.
Top-down plasticity
n order to study cortical layout, O’Leary’s team altered a regulatory gene, Pax6, in the cortex in mice. In response, S1 became much smaller, demonstrating that Pax6 regulates its development. They found that the shrinkage in S1 subsequently affected other regions of the brain that feed sensory information into the cortex.
But more interestingly, it also altered the body maps in these subcortical brain regions, overturning the idea that once established, these brain regions could only be changed by external experience. They dubbed this previously unknown phenomenon “top-down plasticity.”
“Top-down plasticity complements in a reverse fashion the well-known bottom-up plasticity induced by sensory deprivation,” says O’Leary.
Thalamus — main switching station for sensory information
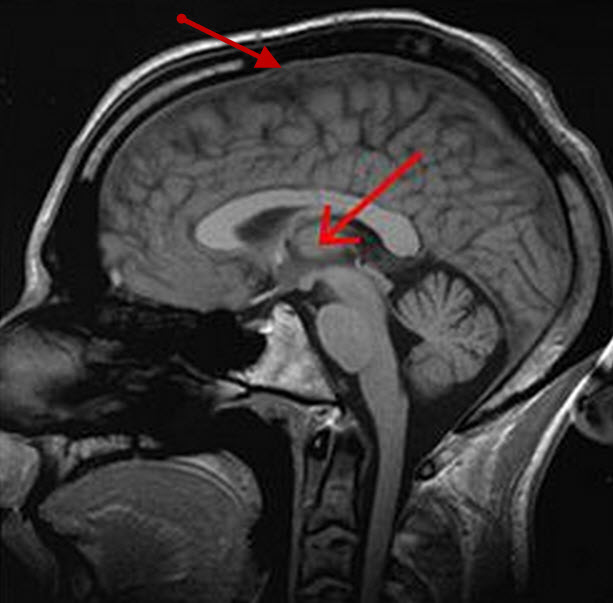
Cortex (top arrow) and thalamus (middle arrow) (credit: Wikimedia Commons)
Normally, the body map in S1 cortex mirrors similar body maps in the thalamus, the main switching station for sensory information, which transmits somatosensation from the body periphery to the S1 cortex through outgoing neural “wires” known as axons.
In the newly discovered top-down plasticity, when S1 was made smaller, the sensory thalamus that feeds into it is also subsequently reduced in size.
But the story has a more intriguing twist. “According to our present knowledge about the development of sensory circuits, we anticipated that all body representations in S1 would be equally affected when S1 was made smaller,” says O’Leary.
“It was a surprise to us that not only was the body map smaller, but some parts of it were completely missing. The specific deletion of parts of the body map is controlled by exaggerated competition for cortical resources dictated by S1 size and played out between the connections from thalamic neurons that form these maps in the cortex.”
“To put it in lay terms, ‘If you snooze, you lose,'” adds Andreas Zembrzycki, a postdoctoral researcher in O’Leary’s lab. “Axons that differentiate later are preferentially excluded from the smaller S1 leading to the specific deletion of the body parts that they represent.”
“The essential point about top-down plasticity is that altering the size and patterning of sensory cortex results in matching alterations in sensory thalamus through the selective death of thalamic neurons that normally would represent body parts absent from S1.”
Aberrant wiring of the brain
“Therefore, a downstream part of the brain is repatterned to match the architecture in S1, resulting in aberrant wiring of the brain that has important implications for sensory perception and function. For example, autistics have very robust abnormalities in touching and other features of somatosensation.”
O’Leary and Zembrzycki believe that this process provides significant insights into the development of autism and other neural disorders. “One of the hallmarks of the autistic brain early in development is the area profile seems to be abnormal, with for example, the frontal cortex being enlarged, while the overall cortex keeps its normal size,” says O’Leary.
“It is implicit then that other cortical areas positioned behind the frontal areas, such as S1, would be reduced in size, and thalamus would exhibit defects that match those in sensory cortex, as has been shown to be the case in autistic patients.”
Other researchers on the study were Shen-Ju Chou of the Salk Institute and Ruth Ashery-Padan and Anastassia Stoykova of the Max Planck Institute for Biophysical Chemistry, Goettingen, Germany.
This work was supported by the National Institutes of Health and the Vincent J.Coates Chair of Molecular Neurobiology.