Brain ‘noise’ found to nurture synapses
May 8, 2014
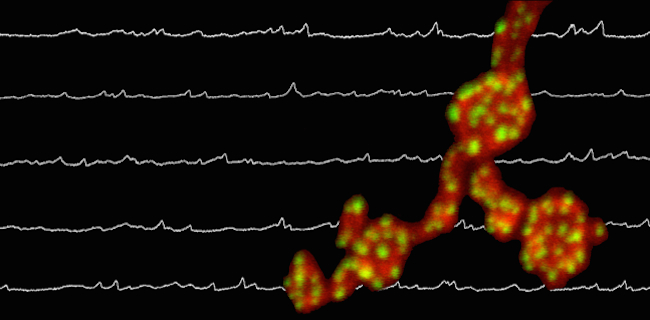
A developing Drosophila synapse superimposed over electrophysiology recordings of minis (credit: Lab of Brian McCabe, PhD/ Columbia University Medical Center)
A long-overlooked form of neuron-to-neuron communication called “miniature neurotransmission” plays an essential role in the development of synapses, a study by researchers at Columbia University Medical Center (CUMC) has shown.
The findings, made in fruit flies, raise the possibility that abnormalities in miniature neurotransmission may contribute to neurodevelopmental diseases. The findings were published in the journal Neuron.
The primary way in which neurons communicate with each another is through “evoked neurotransmission.” This process begins when an electrical signal, or action potential, is transmitted along a long, cable-like extension of the neuron called an axon. Upon reaching the axon’s terminus, the signal triggers the release of chemicals called neurotransmitters across the synapse.
Finally, the neurotransmitters bind to and activate receptors of the neuron on the other side of the synapse. Neurotransmitters are packaged together into vesicles, which are released by the hundreds, if not thousands, with each action potential. Evoked neurotransmission was first characterized in the 1950s by Sir Bernard Katz and two other researchers, who were awarded the 1970 Nobel Prize in Physiology or Medicine for their efforts.
“Dr. Katz also found that even without action potentials, lone vesicles are released now and then at the synapse,” said study leader Brian D. McCabe, PhD, assistant professor of pathology and cell biology and of neuroscience in the Motor Neuron Center. “These miniature events — or minis — have been found at every type of synapse that has been studied. However, since minis don’t induce neurons to fire, people assumed they were inconsequential, just background noise.”
Recent cell-culture studies, however, have suggested that minis do have some function and even their own regulatory mechanisms. “This led us to wonder why there would be such complicated mechanisms for regulating something that was just noise,” said Dr. McCabe.
To learn more about minis, the CUMC team devised new genetic tools to selectively up- or down-regulate evoked and miniature neurotransmission in fruit flies (a commonly used model organism for neuronal function and development). This was the first study to identify a unique role for minis in an animal model.
The researchers found that when both types of neurotransmission were blocked, synapse development was abnormal. However, inhibiting or stimulating evoked neurotransmission alone had no effect on synaptic development. “But when we blocked minis, synapses failed to develop,” said Dr. McCabe, “and when we stimulated the release of more minis, synapses got bigger.”
The study also showed that minis regulate synapse development by activating a signaling pathway in neurons involving Trio and Rac1 proteins in presynaptic neurons. These proteins are also found in humans.
It remains to be seen exactly how minis are exerting their effects. “Parallel communication occurs in computer networks,” Dr. McCabe said. “Computers communicate primarily by sending bursts of data bundled into packets. But individual computers also send out pings, or tiny electronic queries, to determine if there is a connection to other computers. Similarly, neurons may be using minis to ping connected neurons, saying in effect, ‘We are connected and I am ready to communicate.’”
The researchers are currently looking into whether minis have a functional role in the mature nervous system. If so, it’s possible that defects in minis could contribute to neurodegenerative disease.
Abstract of Neuron paper
- Miniature, but not evoked, neurotransmission is required for synapse development
- Miniature neurotransmission bidirectionally regulates synaptic terminal maturation
- Miniature events signal locally through the GEF Trio and the GTPase Rac1
- Miniature neurotransmission has unique and essential functions in vivo
Miniature neurotransmission is the transsynaptic process where single synaptic vesicles spontaneously released from presynaptic neurons induce miniature postsynaptic potentials. Since their discovery over 60 years ago, miniature events have been found at every chemical synapse studied. However, the in vivo necessity for these small-amplitude events has remained enigmatic. Here, we show that miniature neurotransmission is required for the normal structural maturation of Drosophila glutamatergic synapses in a developmental role that is not shared by evoked neurotransmission. Conversely, we find that increasing miniature events is sufficient to induce synaptic terminal growth. We show that miniature neurotransmission acts locally at terminals to regulate synapse maturation via a Trio guanine nucleotide exchange factor (GEF) and Rac1 GTPase molecular signaling pathway. Our results establish that miniature neurotransmission, a universal but often-overlooked feature of synapses, has unique and essential functions in vivo.