Cell-free protein synthesis device is potential lifesaver
January 6, 2016
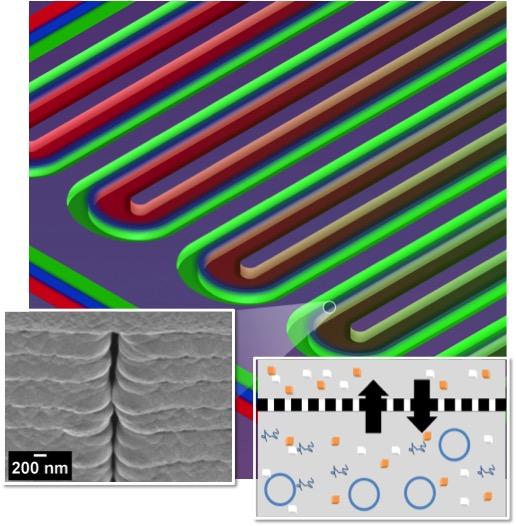
This section of a serpentine channel reactor shows the parallel reactor and feeder channels separated by a nanoporous membrane. Left: a single nanopore viewed from the side; right: a diagram of metabolite exchange across the membrane. (credit: ORNL)
Oak Ridge National Laboratory scientists have developed a device that uses microfabricated bioreactors to produce therapeutic proteins for medicines and biopharmaceuticals. These miniature factories are cell-free, eliminating the need to maintain a living system, which radically simplifies the process and lowers cost, and makes the device easily adaptable for use in isolated locations and at disaster sites.
On-demand, point-of-care therapeutic protein synthesis requires that a dose of protein be produced and purified quickly. “With this approach, we can produce more protein faster, making our technology ideal for point-of-care use,” said team co-leader Scott Retterer of the lab’s Biosciences Division. It can produce proteins “when and where you need them, bypassing the challenge of keeping the proteins cold during shipment and storage.”
The key to the cell-free reactions in the new bioreactor is a permeable nanoporous membrane and serpentine (snake-like) design, made using a combination of electron-beam lithography and advanced material-deposition processes.
The long serpentine channels allow for exchange of materials between parallel reactor and feeder channels. With this approach, the team can control the exchange of metabolites, energy, and species that inhibit production of the desired protein. The design also extends reaction times and improves yields.
“We show that the microscale bioreactor design produces higher protein yields than conventional tube-based batch formats and that product yields can be dramatically improved by facilitating small molecule exchange with the dual-channel bioreactor,” the authors wrote in their paper, published in the journal Small.
The researchers also note that on-demand biologic synthesis would aid production of drugs that are costly to mass-produce, including orphan drugs and personalized medicines.
Abstract of Toward Microfluidic Reactors for Cell-Free Protein Synthesis at the Point-of-Care
Cell-free protein synthesis (CFPS) is a powerful technology that allows for optimization of protein production without maintenance of a living system. Integrated within micro and nanofluidic architectures, CFPS can be optimized for point-of-care use. Here, the development of a microfluidic bioreactor designed to facilitate the production of a single-dose of a therapeutic protein, in a small footprint device at the point-of-care, is described. This new design builds on the use of a long, serpentine channel bioreactor and is enhanced by integrating a nanofabricated membrane to allow exchange of materials between parallel “reactor” and “feeder” channels. This engineered membrane facilitates the exchange of metabolites, energy, and inhibitory species, and can be altered by plasma-enhanced chemical vapor deposition and atomic layer deposition to tune the exchange rate of small molecules. This allows for extended reaction times and improved yields. Further, the reaction product and higher molecular weight components of the transcription/translation machinery in the reactor channel can be retained. It has been shown that the microscale bioreactor design produces higher protein yields than conventional tube-based batch formats, and that product yields can be dramatically improved by facilitating small molecule exchange within the dual-channel bioreactor.