Cellular reprogramming turns back the aging clock in mice
December 18, 2016
This cartoon depicts turning back the aging clock through cellular regeneration of progeria mice (credit: Juan Carlos Izpisua Belmonte Lab/Salk Institute)
Salk Institute scientists have extended the average lifespan of live mice by 30 percent, according to a study published December 15 in Cell. They did that by rolling back the “aging clock” to younger years, using cellular reprogramming.
The finding suggests that aging is reversible by winding back an animal’s biological clock to a more youthful state and that lifespan can be extended. While the research does not yet apply directly to humans, it promises to lead to improved understanding of human aging and the possibility of rejuvenating human tissues.
To achieve this, the scientists worked with “progeria” mouse models — mice that had been genetically modified to carry a mutation that leads to premature aging (allowing the aging effects to be isolated and studied).*
Rather than attempting to correct the genetic mutations that cause premature aging (a difficult challenge), the Salk team instead focused on restoring the epigenome (a system of chemical marks on the genome that control which genes are or are not expressed as proteins)**.
Partial reprogramming avoids tumors and aging effects
To wind back the clock, the scientists first reprogrammed progeria mouse adult cells (such as skin cells) into an induced pluripotent stem (iPS) cells*** — an early embryonic state in which the cell is not yet specialized (“differentiated”) and can be programmed to perform specific functions, such as becoming part of the skin or heart.
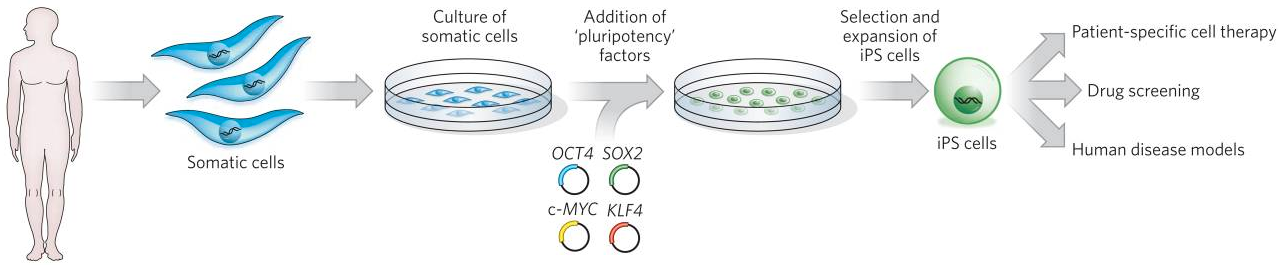
To generate iPS cells, fibroblasts (or another type of adult somatic cell) are transduced with retroviruses encoding four pluripotency factors (SOX2, KLF4, c-MYC and OCT4). These differentiated cells can be used in disease models (such as progeria) for studying the molecular basis of a broad range of human diseases that are otherwise difficult to study. (credit: Yamanaka S. et al./Nature)
The scientists did that by inducing (modifying) the expression of four specific “epigenetic marks” called “Yamanaka factors” in the iPS cells, with the goal of allowing the cells to grow into adult cells without defects.
Researchers have previously done that successfully with cells in vitro (in a test tube), allowing these factors to be expressed for the needed 2 to 3 weeks for cells. But when they tried that in vivo (in live mice), it resulted in mice that later either died immediately or developed extensive tumors.
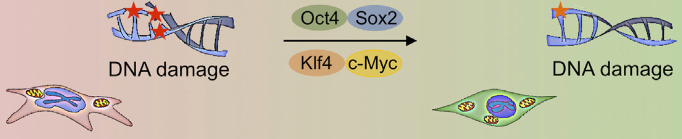
Partial cellular reprogramming with the four Yamanaka factors (Oct4, Sox2, Klf4, c-Myc) erases cellular markers of aging in progeria mouse cells (credit: Alejandro Ocampo et al./Cell)
So instead, the Salk team used partial in vivo cellular reprogramming. They induced expression of Yamanaka factors for just 2 to 4 days instead of 2 to 3 weeks. Instead of resulting in tumors or death, that prolonged the mice lifespan. An in vitro experiment also showed reduced DNA damage accumulation and restored nuclear structure.
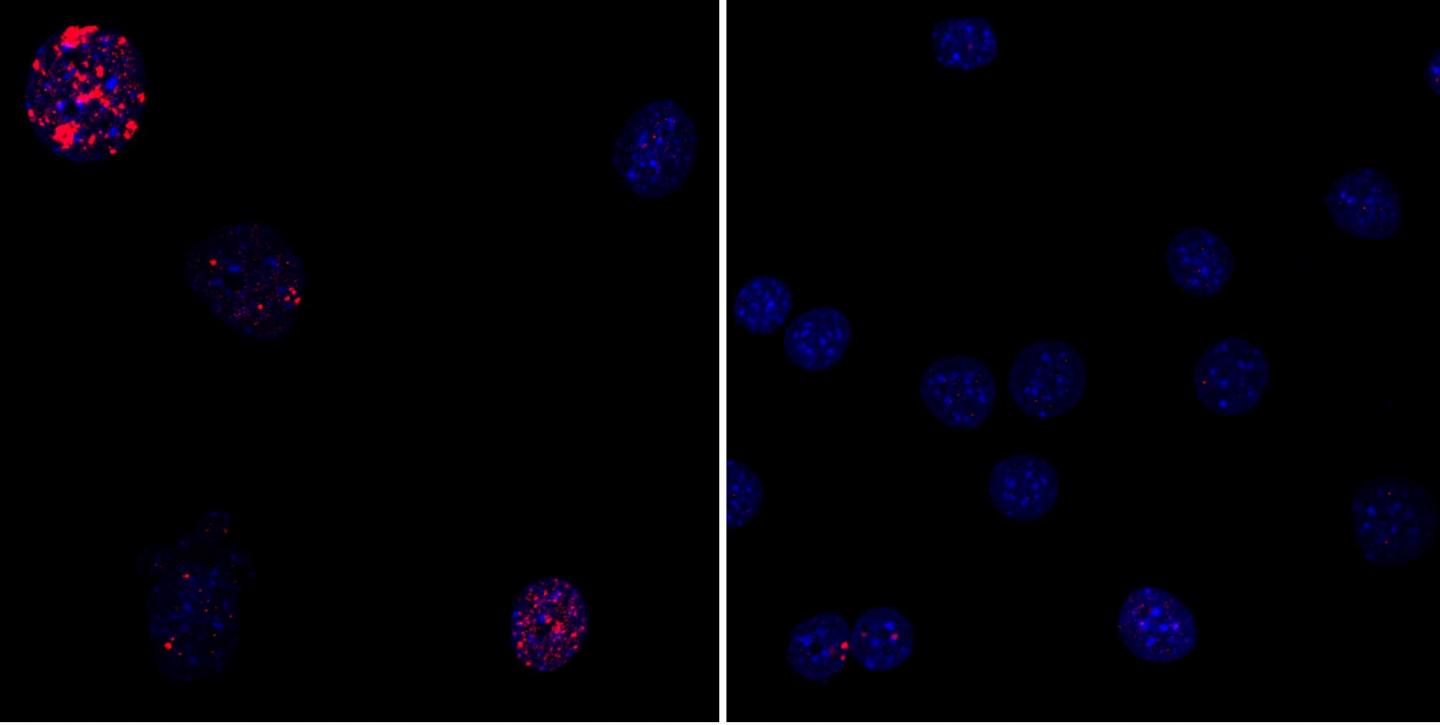
This image depicts the discovery by Salk Institute researchers that partial cellular reprogramming reversed cellular signs of aging such as accumulation of DNA damage. (Left) Progeria mouse fibroblast cells; (Right) Progeria mouse fibroblast cells rejuvinated by partial reprogramming. (credit: Juan Carlos Izpisua Belmonte Lab/Salk Institute)
“These changes are the result of epigenetic remodeling in the cell,” notes lead investigator Juan Carlos Izpisua Belmonte, a professor in the Salk Institute of Biological Science’s Gene Expression Laboratory.
Several organs improved. For instance, tissue from skin, spleen, kidney and stomach all had improved appearance when inspected under the microscope. The cardiovascular system, which often fails and causes early death in these prematurely aging mice, also showed improvements in structure and function.
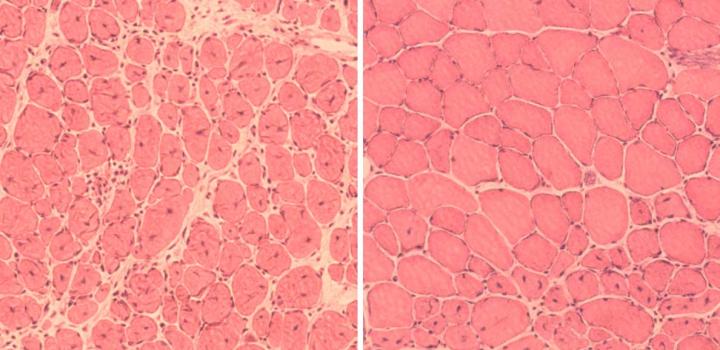
This image shows the discovery by researchers that induction of partial cellular reprogramming improved muscle regeneration in aged mice. (Left) Impaired muscle repair in aged mice; (Right) Improved muscle regeneration in aged mice subjected to reprogramming. (credit: Juan Carlos Izpisua Belmonte Lab/Salk Institute)
The team also tested partial reprogramming in mouse models that had injury, resulting in enhanced regeneration of muscle tissue and pancreas beta cells (which store and release insulin).
Next steps will involve learning more about how the epigenome changes during partial reprogramming. “We need to go back and explore which marks are changing and driving the aging process,” says Belmonte.
This study was supported by the G. Harold and Leila Y. Mathers Charitable Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, The Glenn Foundation, Universidad Católica San Antonio de Murcia (UCAM), and Fundación Dr. Pedro Guillen.
* Mice do not perfectly mimic human aging. Researchers generate and use mouse models of premature aging by introducing mutations responsible for these human diseases in mouse.
** Epigenetic marks in the DNA regulate and protect the genome. Some marks turn on specialized functions, such as skin cell machinery in a skin cell; others turn off mechanisms that aren’t needed, such as liver cell machinery in the skin. They are modified over a lifetime in response to environmental changes, and have been proposed as drivers of aging.
*** Induced pluripotent stem cells (iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from adult cells. The iPSC technology was pioneered by Shinya Yamanaka’s lab in Kyoto, Japan, who showed in 2006 that the introduction of four specific genes encoding transcription factors could convert adult cells into pluripotent stem cells. He was awarded the 2012 Nobel Prize in Physiology or Medicine with Sir John Gurdon.
Salk Institute | Salk scientists reverse signs of aging in mice
Abstract of In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming
Aging is the major risk factor for many human diseases. In vitro studies have demonstrated that cellular reprogramming to pluripotency reverses cellular age, but alteration of the aging process through reprogramming has not been directly demonstrated in vivo. Here, we report that partial reprogramming by short-term cyclic expression of Oct4, Sox2, Klf4, and c-Myc (OSKM) ameliorates cellular and physiological hallmarks of aging and prolongs lifespan in a mouse model of premature aging. Similarly, expression of OSKM in vivo improves recovery from metabolic disease and muscle injury in older wild-type mice. The amelioration of age-associated phenotypes by epigenetic remodeling during cellular reprogramming highlights the role of epigenetic dysregulation as a driver of mammalian aging. Establishing in vivo platforms to modulate age-associated epigenetic marks may provide further insights into the biology of aging.