Changes in bioelectric signals trigger formation of new organs; regenerative medicine implications
December 9, 2011
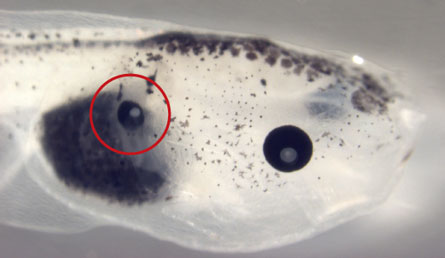
A tadpole with an extra eye growing in its gut (indicated by red circle), a result of manipulating electrical signals in gut cells. (credit: Sherry Aw, Vaibhav Pai, M. Levin)
In a major discovery, biologists at Tufts University were able to cause tissue to grow a new organ by simply altering the membrane voltage gradients of cells: they caused tadpoles to grow eyes outside of the head area.
These findings break new ground in the field of biomedicine because they identify an entirely new control mechanism that can be used to induce the formation of complex organs for transplantation or regenerative medicine applications, according to Michael Levin, Ph.D., professor of biology and director of the Center for Regenerative and Developmental Biology at Tufts University’s School of Arts and Sciences.
What’s especially interesting about this is that in research starting in 1937, Dr. Harold S. Burr, Professor Emeritus, Anatomy at Yale University School of Medicine, discovered that abnormal growth (such as cancer) was preceded by the appearance of abnormal voltage gradients in an organ. In a related discovery, Tufts biologists were able to control the incidence of abnormal eyes by manipulating the voltage gradient in the embryo.
The researchers achieved the most surprising results when they manipulated membrane voltage of cells in the tadpole’s back and tail, well outside of where the eyes could normally form. “The hypothesis is that for every structure in the body there is a specific membrane voltage range that drives organogenesis,” said Tufts post-doctoral fellow Vaibhav P. Pai, Ph.D.
Pai noted, “These were cells in regions that were never thought to be able to form eyes. This suggests that cells from anywhere in the body can be driven to form an eye.” To do this, they changed the voltage gradient of cells in the tadpoles’ back and tail to match that of normal eye cells. The eye-specific gradient drove the cells in the back and tail — which would normally develop into other organs — to develop into eyes.
“These results reveal a new regulator of eye formation during development, and suggest novel approaches for the detection and repair of birth defects affecting the visual system,” he said. “Aside from the regenerative medicine applications of this new technique for eyes, this is a first step to cracking the bioelectric code.”
Signals Turn On Eye Genes
From the outset of their research, the Tufts’ biologists wanted to understand how cells use natural electrical signals to communicate in their task of creating and placing body organs. In recent research, Tufts biologist Dany S. Adams showed that bioelectrical signals are necessary for normal face formation in the Xenopus (frog) embryos. In the current set of experiments, the Levin lab identified and marked hyperpolarized (more negatively charged) cell clusters located in the head region of the frog embryo.
They found that these cells expressed genes that are involved in building the eye called Eye Field Transcription Factors (EFTFs). Sectioning of the embryo through the developed eye and analyzing the eye regions under fluorescence microscopy showed that the hyperpolarized cells contributed to development of the lens and retina. The researchers hypothesized that these cells turned on genes that are necessary for building the eye.
Electric Properties of Cells Can Be Manipulated to Generate Specific Organs
The researchers achieved most surprising results when they manipulated membrane voltage of cells in the tadpole’s back and tail, well outside of where the eyes could normally form.
“The hypothesis is that for every structure in the body there is a specific membrane voltage range that drives organogenesis,” said Pai. “By using a specific membrane voltage, we were able to generate normal eyes in regions that were never thought to be able to form eyes. This suggests that cells from anywhere in the body can be driven to form an eye.”
Levin and his colleagues are pursuing further research, additionally targeting the brain, spinal cord, and limbs. The findings, he said “will allow us to have much better control of tissue and organ pattern formation in general. We are developing new applications of molecular bioelectricity in limb regeneration, brain repair, and synthetic biology.”
Changing the Signals Lead to Defects
Changing the bioelectric code, or depolarizing these cells, also affected normal eye formation. They injected the cells with mRNA encoding ion channels, which are a class of gating proteins embedded in the membranes of the cell. Like gates, each ion channel protein selectively allows a charged particle to pass in and out of the cell.
Using individual ion channels, the researchers changed the membrane potential of these cells. This affected expression of EFTF genes, causing abnormalities to occur: Tadpoles from these experiments were normal except that they had deformed or no eyes at all.
Further, the Tufts biologists were also able to show that they could control the incidence of abnormal eyes by manipulating the voltage gradient in the embryo. “Abnormalities were proportional to the extent of disruptive depolarization,” said Pai. “We developed techniques to raise or lower voltage potential to control gene expression.”
Ref.: Vaibhav P. Pai et al., Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis, Development, 2011 [doi: 10.1242/dev.073759]