Changing behavior with synapse engineering
September 16, 2015
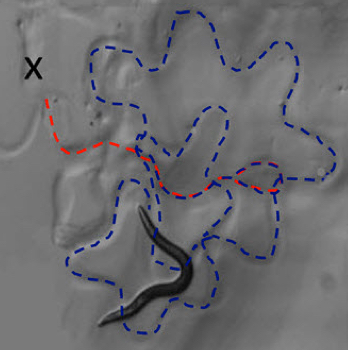
The worm turns: injecting a transgenic nematode worm with tyramine induces the worm to switch from forward locomotion (dashed red line, starting at X) to backward locomotion (dashed blue line) (credit: Jennifer K. Pirri et al./PLOS Biology)
In 1963, Yale professor of physiology and psychiatry Dr. Jose Delgado implanted an stimulating electrode in the caudate nucleus of a fighting bull, bravely jumped into the bullring, and stopped the animal in its tracks by remotely activating the electrode. Now UMass Medical School scientists have taken neural control precision down to the synapse level, reversing a C. elegans (nematode) worm’s head position or locomotion direction by simply switching one of its synapses (neuron-to-neuron connections) from inhibitory to excitatory (“off” to “on”).
The research, published in an open-access paper in PLOS Biology, offers a new approach for studying the neural circuits that govern behavior and has implications for refining the connectome (neural roadmap), which is important for understanding how the 100 billion neurons and quadrillion (1015) synapse process information and control behavior.
Currently, the connectome doesn’t include information about the activity of specific neurons or the signals they transmit. The complexity of the human brain makes it almost impossible to address questions such as how stable are neural circuits in the brain and how their wiring constrains the flow of information or the behaviors they control.
So Mark Alkema, PhD, associate professor of neurobiology, turned to the nematode C. elegans. A tiny worm with only 302 neurons, it is the only animal whose neural roadmap has been completely defined.
Worm mind control
Alkema and colleagues wanted to determine if flipping the sign (inhibitory or excitatory) of a synapse in the worm’s brain was enough to reverse a behavior. To do this, they analyzed the touch response that allows C. elegans to escape from carnivorous fungi that use threadlike nooses to catch nematodes. During this escape response, neurotransmitters in C. elegans are released that activate an inhibitory ion channel. This causes the worm to relax its head and quickly reverse direction away from the predator.
Synapse firing is determined by the charge of the ions that flow through channels. So they replaced the inhibitory ion channel with an excitatory version of the channel in a live nematode. “Surprisingly, the engineered channel does not affect the worms’ development and is properly incorporated into the neural circuits of the worm brain,” said Alkema. “Cells that are normally inhibited in the brain now get activated.”
“What was most striking is that we were able to completely reverse behavior by simply switching the sign of a synapse in the neural network,” explained Alkema. “Now the animal contracts its head and tends to move forward in response to touch. This suggests that the neural wiring diagram is remarkably stable and allows these types of changes.”
“Our studies indicate that switching the sign of a synapse not only provides a novel synthetic mechanism to flip behavioral output but could even be an evolutionary mechanism to change behavior,” said Alkema. “As we start to unravel the complexity and design of the neural network, it holds great promise as a novel mechanism to test circuit function or even design new neural circuits in vivo.”
Abstract of A Change in the Ion Selectivity of Ligand-Gated Ion Channels Provides a Mechanism to Switch Behavior
Behavioral output of neural networks depends on a delicate balance between excitatory and inhibitory synaptic connections. However, it is not known whether network formation and stability is constrained by the sign of synaptic connections between neurons within the network. Here we show that switching the sign of a synapse within a neural circuit can reverse the behavioral output. The inhibitory tyramine-gated chloride channel, LGC-55, induces head relaxation and inhibits forward locomotion during the Caenorhabditis elegans escape response. We switched the ion selectivity of an inhibitory LGC-55 anion channel to an excitatory LGC-55 cation channel. The engineered cation channel is properly trafficked in the native neural circuit and results in behavioral responses that are opposite to those produced by activation of the LGC-55 anion channel. Our findings indicate that switches in ion selectivity of ligand-gated ion channels (LGICs) do not affect network connectivity or stability and may provide an evolutionary and a synthetic mechanism to change behavior.