Chemical makes blind mice instantly see — without invasive surgery
July 29, 2012
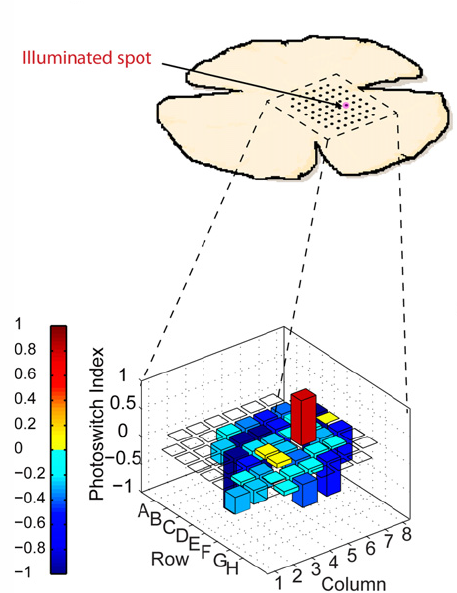
Only the specific targeted 60-micron area of the retina in AAQ-treated blind mice fires when stimulated by flashes of violet light (credit: A. Polosukhina et al./Neuron)
A chemical called AAQ that temporarily restores some vision to blind mice has been discovered by University of California, Berkeley scientists in collaboration with researchers at the University of Munich and University of Washington in Seattle.
The discovery could eventually help those with retinitis pigmentosa, a genetic disease that is the most common inherited form of blindness, as well as age-related macular degeneration, the most common cause of acquired blindness in the developed world.
In both diseases, the light sensitive cells in the retina — the rods and cones — die, leaving the eye without functional photoreceptors.
AAQ acts by making the retinal ganglion cells (RGCs) — which are normally blind — sensitive to light, said lead researcher Richard Kramer, UC Berkeley professor of molecular and cell biology. AAQ (acrylamide-azobenzene-quaternary ammonium) is a “photoswitch” that binds to protein ion channels on the surface of retinal cells.
When switched on by light, AAQ alters the flow of potassium ions through the channels and activates RGCs much the way rods and cones are activated by light.
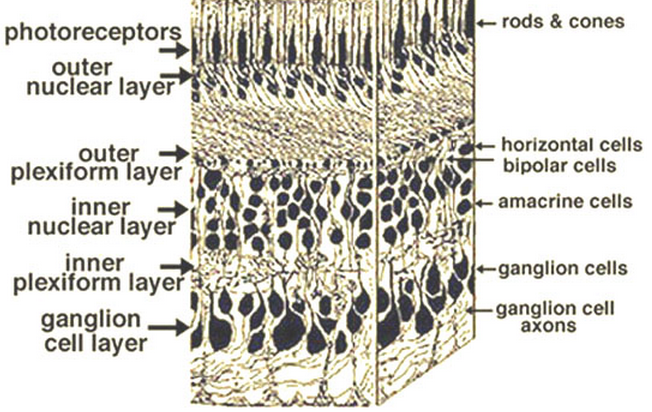
Retina layers (light enters from the bottom). Axons from photosensitive retinal ganglion cells (bottom) extend via the optic nerve bundle to the brain. (Credit: Webvision/Creative Commons)
There are 1.2 to 1.5 million RGCs in the eye; they conduct signals from rods and cones to the brain. The goal of vision restoration research is to recreate a natural visual scene as closely as possible with the activity of RGCs, the researchers say.
Testing the effects of light on blind mice
The blind mice in the experiment had genetic mutations that made their rods and cones die within months of birth and inactivated other photopigments in the eye.
After injecting very small amounts of AAQ into the eyes of the blind mice, Kramer and his colleagues confirmed that they had restored light sensitivity because the mice’s pupils contracted in bright light, and the mice showed light avoidance, a typical rodent behavior impossible without the animals being able to see some light:
The eye of the untreated mouse on the left shows no response to light, while the pupil of the mouse on the right, which was injected with the chemical, contracts in light (credit: A. Polosukhina et al./Neuron; video post: Giulio Prisco/KurzweilAI)
Kramer hopes to conduct more sophisticated vision tests in rodents injected with the next generation of the compound.
“The photoswitch approach offers real hope to patients with retinal degeneration,” Van Gelder said. “We still need to show that these compounds are safe and will work in people the way they work in mice, but these results demonstrate that this class of compound restores light sensitivity to retinas blind from genetic disease.”
Safer, more controllable
There are three current technologies being evaluated for restoring sight to people whose rods and cones have died. They are highly invasive and irreversible:
- Surgical injection of stem cells to regenerate the rods and cones.
- Optogenetics, that is, gene therapy using a virus to insert a photoreceptor gene into blind neurons to make them sensitive to light. It may result in inflammatory responses.
- Installation of electronic prosthetic devices, such as a small light-sensitive retinal chip with electrodes that stimulate a few hundred RGCs (compared to more than 1.2 million RGCs with AAQ) and a camera. Several dozen people already have retinal implants and have had rudimentary, low vision restored, Kramer said. However, these devices require invasive surgery. The $99,350 Second Sight Argus II prosthesis is limited to 64 pixels, but it’s the closest product to FDA approval (Second Sight Medical Products Inc. announced on July 20 that a FDA Ophthalmic Devices Advisory Panel has been scheduled on September 28 to review the data presented in the company’s Humanitarian Device Exemption market approval application for its Argus II Retinal Prosthesis System). An improved implant is also in the pre-clinical testing stage, and the company also hopes to move the site of stimulation downstream to the visual cortex, Second Sight spokesman Brian Mech told KurzweilAI. There’s also a prosthesis called Bio-Retina being developed by Nano Retina, that they claim will provide 576 pixels.
“A several month supply of AAQ could be packaged into an tiny device inserted into the eye cavity like those currently used for long-term steroid treatment of ocular inflammation,” the researchers explain in their Neuron paper.
Eight years ago, Kramer, chemist Dirk Trauner (a former UC Berkeley chemist now at the University of Munich), and their colleagues developed an optogenetic technique to chemically alter potassium ion channels in blind neurons so that a photoswitch could latch on. Potassium channels normally open to turn a cell off, but with the attached photoswitch, they were opened when hit by ultraviolet light and closed when hit by green light, thereby activating and deactivating the neurons.
Subsequently, Trauner synthesized AAQ, a photoswitch that attaches to potassium channels without the need to genetically modify the channel.
New versions of AAQ now being tested are better, Kramer said. They activate neurons for days rather than hours using blue-green light of moderate intensity, and these photoswitches naturally deactivate in darkness, so that a second color of light is not needed to switch them off. “This is what we are really excited about,” he said.
“This is a major advance in the field of vision restoration,” said co-author Dr. Russell Van Gelder, an ophthalmologist and chair of the Department of Ophthalmology at the University of Washington, Seattle.
The work was supported by the National Eye Institute of the National Institutes of Health (EY018957 & EY003176) and Research to Prevent Blindness.