Computers found more accurate than doctors in breast-cancer diagnosis
November 10, 2011
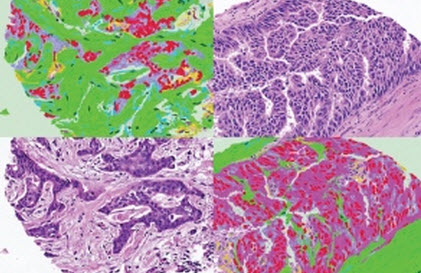
C-Path scans stained tumor images (bottom left and top right) and labels them (top left and bottom right) to predict which tumors need more aggressive treatment (credit: Science/AAAS)
Computer analyses of breast cancer microscopic images were found more accurate than those conducted by humans, computer scientists at the Stanford School of Engineering and pathologists at the Stanford School of Medicine report.
The researchers’ Computational Pathologist (C-Path) is a machine-learning-based method for automatically analyzing images of cancerous tissues and predicting patient survival.
Since 1928, the way breast cancer characteristics are evaluated and categorized has remained largely unchanged: done by hand, under a microscope. Pathologists examine the tumors visually and score them according to a scale first developed eight decades ago. These scores help doctors assess the type and severity of the cancer and calculate the patient’s prognosis and course of treatment.
Medical science has long used three specific features for evaluating breast cancer cells: what percentage of the tumor is comprised of tube-like cells, the diversity of the nuclei in the outermost (epithelial) cells of the tumor and the frequency with which those cells divide (a process known as mitosis). These three factors are judged by sight with a microscope and scored qualitatively to stratify breast cancer patients into three groups that predict survival rates.
Training C-Path: 6,642 cellular factors
To train C-Path, the researchers used existing tissue samples taken from patients whose prognosis was known. By comparing results against the known data, the computers adapted their models to better predict survival and gradually figured out what features of the cancers matter most and which matter less in predicting survival.
“Pathologists have been trained to look at and evaluate specific cellular structures of known clinical importance, which get incorporated into the grade. However, tumors contain innumerable additional features, whose clinical significance has not previously been evaluated,” said Andrew Beck, MD, a doctoral candidate in biomedical informatics and the paper’s first author.
C-Path assesses 6,642 cellular factors. C-Path yielded results that were a statistically significant improvement over human-based evaluation. What’s more, the computers identified structural features in cancers that matter as much or more than those that pathologists have focused on traditionally. In fact, they discovered that the characteristics of the cancer cells and the surrounding cells, known as the stroma, were both important in predicting patient survival.
C-Path could improve the accuracy of prognoses for all breast cancer victims. It could, likewise, improve the screening of pre-cancerous cells that could help many women avoid cancer altogether. It might even be applied to predict the effectiveness of various forms of treatment and drug therapies.
“If we can teach computers to look at a tumor tissue sample and predict survival, why not train them to predict from the same sample which courses of treatment or drugs a given patient might respond to best? Or even to look at samples of non-malignant cells to predict whether these benign tissues will turn cancerous,” said Daphne Koller, PhD, professor of computer science and senior author of the paper. “This is personalized medicine.”
Ref.: Andrew H. Beck, et al., Systematic Analysis of Breast Cancer Morphology Uncovers Stromal Features Associated with Survival, Science Translational Medicine, 2011; [DOI: 10.1126/scitranslmed.3002564]