Controlling genes with mental states to release drugs
November 18, 2014
Mental states control a wireless-powered near-infrared LED, which troggers production of a molecule in a reaction chamber (credit: Martin Fussenegger/ETH Zurich)
ETH Zurich researchers have developed a novel gene regulation method that allows specific brainwaves to control gene expression (conversion of a gene into a protein) for therapeutic purposes.
The concept is a thought-controlled implant that could one day help combat neurological diseases, such as chronic headaches, back pain, and epilepsy.
An EEG-based BCI (brain-controlled interface) would detect the patient’s related brainwave patterns, which would be used to trigger a gene switch that would modulate the creation of certain chemical agents (such a drugs).
The experimental system is described in the journal Nature Communications. The implant was initially tested in cell cultures and mice, and controlled by the brainwaves of various test subjects.
Wireless transmission from brain to implant
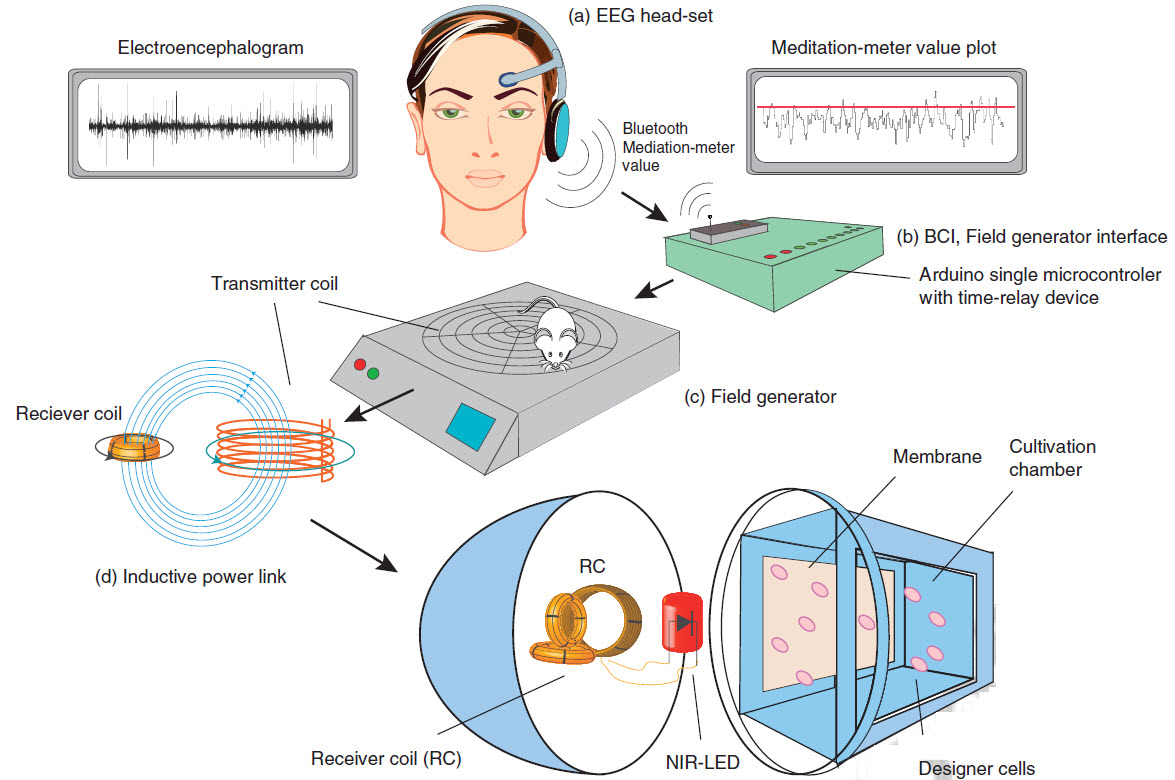
This diagram for the experiment shows how the system interprets and transforms specific brainwaves to power a near-infrared LED and cause a gene to trigger release of a protein (credit: Folcher M et al./Nature Communications)
The system uses an EEG headset (MindSet from NeuroSky) was used in the experiment) to detect brainwaves that are wirelessly transmitted via Bluetooth to a microcontroller (Arduino Uno). It controls an inductive field generator (transmitter) that wirelessly powers an inductively linked optogenetic near-infrared* LED light for defined periods of time (60 min/30 s). The light illuminates a culture chamber containing genetically modified cells, causing them to produce the desired protein. The protein then diffuses from the culture chamber of the implant into the mouse’s bloodstream. For the tests, the researchers used the SEAP protein, an easy-to-detect human model protein.
To regulate the quantity of released protein, three mental states were used: concentration, meditation, and biofeedback. Test subjects who played Minecraft on the computer (were concentrating) induced average SEAP values in the bloodstream of the mice. When completely relaxed (meditation), the researchers recorded very high SEAP values in the test animals. For biofeedback, the test subjects observed the LED light of the implant in the body of the mouse and were able to consciously switch the LED light on or off via the visual feedback. This in turn was reflected by the varying amounts of SEAP in the bloodstream of the mice.
“Controlling genes in this way is completely new and is unique in its simplicity,” explains Martin Fussenegger, Professor of Biotechnology and Bioengineering in the ETH Department of Biosystems.
According to the researchers, far into the future, patients may learn to generate specific mental states (for pain relief or locked-in syndrome, for example) to drive therapeutic implants to produce relevant doses of protein pharmaceuticals; or for neurodegenerative disorders such as epilepsy, the system could autonomously produce the chemicals, with closed-loop control.
* Near-infrared light was used because it is generally not harmful to human cells, can penetrate deep into the tissue, and enables the function of the implant to be visually tracked.
Abstract of Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant
Synthetic devices for traceless remote control of gene expression may provide new treatment opportunities in future gene- and cell-based therapies. Here we report the design of a synthetic mind-controlled gene switch that enables human brain activities and mental states to wirelessly programme the transgene expression in human cells. An electroencephalography (EEG)-based brain–computer interface (BCI) processing mental state-specific brain waves programs an inductively linked wireless-powered optogenetic implant containing designer cells engineered for near-infrared (NIR) light-adjustable expression of the human glycoprotein SEAP (secreted alkaline phosphatase). The synthetic optogenetic signalling pathway interfacing the BCI with target gene expression consists of an engineered NIR light-activated bacterial diguanylate cyclase (DGCL) producing the orthogonal second messenger cyclic diguanosine monophosphate (c-di-GMP), which triggers the stimulator of interferon genes (STING)-dependent induction of synthetic interferon-β promoters. Humans generating different mental states (biofeedback control, concentration, meditation) can differentially control SEAP production of the designer cells in culture and of subcutaneous wireless-powered optogenetic implants in mice.