Creating a near-living crystal structure from colloids
February 1, 2013
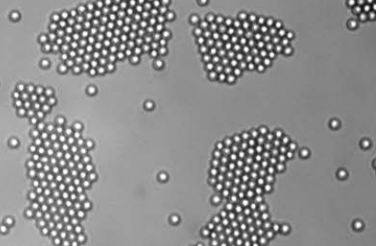
Living crystals assembled from a homogeneous distribution under illumination by blue light. (Credit: Jeremie Palacci et al./Science)
New York University physicists have developed a method for moving microscopic particles, using a blue light to prompt colloids to move and then assemble — much like birds flock and move together in flight.
The method could enhance the design of a range of industrial products, including the architecture of electronics.
The work addresses a fundamental question in nature — what causes flocks and swarms to form and move in a particular way? Schools of fish, colony formations of bacteria, or flocks of birds are examples of how this occurs in living matter.
The researchers focused on making artificial systems exhibit similar activity. They used colloids — small particles suspended within a fluid medium — and discovered the basic organizing principles in natural flocking and how to use this to organize inorganic matter.
Colloidal dispersions are composed of such everyday items such as paint, milk, gelatin, glass, and porcelain. By better understanding driven colloidal self-organization, scientists could harness these particles tocreate new and enhanced materials.
Light-activated self-propelled particles

(D→G): The false colors show the time evolution of particles belonging to different clusters. The clusters are not
static but rearrange, exchange particles, merge (D–F), break apart (E→F) or become unstable and explode (blue
cluster, F→G). (Credit: Jeremie Palacci et al./Science)
To explore this, the research team developed light-activated self-propelled particles, “swimmers,” from the micron-sized particles in solution. To separate the effects of swimming from simple thermal motion, they created a system where the particles turn on and off with application of blue light.
With the light on, the self-propelled random swimmers collide and cluster. The light also triggers a slight chemical attraction and leads the clusters to crystallize and grow until the swimmers turn in separate directions and splinter the crystals. The “living” crystals continually form, swirl, and split. When the light is extinguished, the swimmers stop and the structures dissolve into individual diffusing colloidal particles.
Using the slight magnetism of the particles allows direction of the individual swimmers as well as the crystals. With control of light, magnets, and chemical attraction, these active particles bring biological organization to the materials world.
The research was supported by grants from the National Science Foundation, under the NSF Materials Research Science and Engineering Center (MRSEC) Program (DMR-0820341), the Department of Defense, under its Multidisciplinary Research Program of the University Research Initiative (W911NF-10-1-0518), and NASA under grant award NNX08AK04G.