Designing new ultrasound imaging tools with Lego-like proteins
August 26, 2016
Protein-shelled structures called gas vesicles, illustrated here, can be engineered with Lego-like proteins to improve ultrasound methods. The gas vesicles can help detect specific cell types and create multicolor images. (credit: Barth van Rossum for Caltech)
The next step in ultrasound imaging will let doctors view specific cells and molecules deeper in the body, such as those associated with tumors or bacteria in our gut.
A new study from Caltech outlines how protein engineering techniques might help achieve this milestone. The researchers engineered protein-shelled nanostructures called gas vesicles (which reflect sound waves) to exhibit new properties useful for ultrasound technologies. In the future, these gas vesicles could be administered to a patient to visualize tissues of interest.
The modified gas vesicles were shown to give off more distinct signals (making them easier to image), target specific cell types, and help create color ultrasound images.
“It’s somewhat like engineering with molecular Legos,” says assistant professor of chemical engineering and Heritage Principal Investigator Mikhail Shapiro, who is the senior author of a new paper about the research published in this month’s issue of the journal ACS Nano and featured on the journal’s cover. “We can swap different protein ‘pieces’ on the surface of gas vesicles to alter their targeting properties and to visualize multiple molecules in different colors.”
“Gas vehicle” proteins reflect sound waves
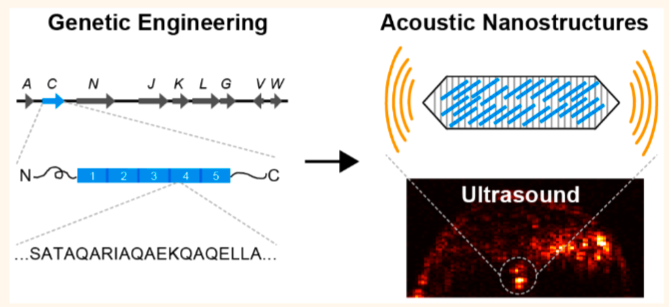
Genetic engineering of gas vesicles — genetically encoded protein nanostructures isolated from buoyant photosynthetic microbes — results in nanostructures with new mechanical, acoustic, surface, and functional properties to enable harmonic, multiplexed, and multimodal ultrasound imaging as well as cell-specific molecular targeting. (credit: Anupama Lakshmanan et al./ACS Nano)
In 2014, Shapiro first discovered the potential use of gas vesicles in ultrasound imaging. These gas-filled structures are naturally occurring in water-dwelling single-celled organisms, such as Anabaena flos-aquae, a species of cyanobacteria that forms filamentous clumps of multicell chains.
The gas vesicles help the organisms control how much they float and thus their exposure to sunlight at the water’s surface. Shapiro realized that the vesicles would readily reflect sound waves during ultrasound imaging, and ultimately demonstrated this using mice.
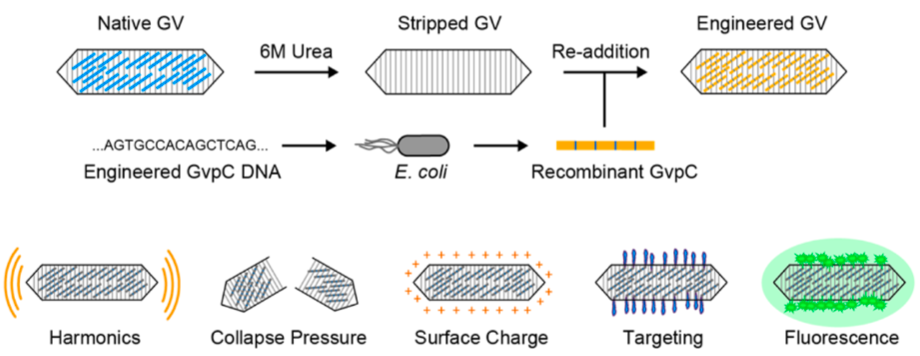
Genetic engineering a type of protein called GvpC (gas vesicle protein C) can be used to modify the properties of acoustic gas-vesicle nanostructures. (credit: Anupama Lakshmanan et al./ACS Nano)
In the latest research, Shapiro and his team set out to give the gas vesicles new properties by engineering gas vesicle protein C, or GvpC, a protein naturally found on the surface of vesicles that gives them mechanical strength and prevents them from collapsing. The protein can be engineered to have different sizes, with longer versions of the protein producing stronger and stiffer nanostructures.
In one experiment, the scientists removed the strengthening protein from gas vesicles and then administered the engineered vesicles to mice and performed ultrasound imaging. Compared to normal vesicles, the modified vesicles vibrated more in response to sound waves, and thus resonated with harmonic frequencies.
Harmonics are created when sound waves bounce around, for instance in a violin, and form new waves with doubled and tripled frequencies. Harmonics are not readily created in natural tissues, making the vesicles stand out in ultrasound images.
In another set of experiments, the researchers demonstrated how the gas vesicles could be made to target certain tissues in the body. They genetically engineered the vesicles to display various cellular targets, such as an amino acid sequence that recognizes proteins called integrins that are overproduced in tumor cells.
Multicolor ultrasound images
The team also showed how multicolor ultrasound images might be created. Conventional ultrasound images appear black and white. Shapiro’s group created an approach for imaging three different types of gas vesicles as separate “colors” based on their differential ability to resist collapse under pressure. The vesicles themselves do not appear in different colors, but they can be assigned colors based on their different properties.
To demonstrate this, the team made three different versions of the vesicles with varying strengths of the GvpC protein. They then increased the ultrasound pressures, causing the variant populations to successively collapse one by one.
As each population collapsed, the overall ultrasound signal decreased in proportion to the amount of that variant in the sample, and this signal change was then mapped to a specific color. In the future, if each variant population targeted a specific cell type, researchers would be able to visualize the cells in multiple colors.
“You might be able to see tumor cells versus the immune cells attacking the tumor, and thus monitor the progress of a medical treatment,” says Shapiro.
The ACS Nano paper, entitled “Molecular Engineering Of Acoustic Protein Nanostructures,” was funded by the National Institutes of Health, the Defense Advanced Research Projects Agency, the Heritage Research Institute for the Advancement of Medicine and Science at Caltech, and the Burroughs Wellcome Fund.
Abstract of Molecular Engineering of Acoustic Protein Nanostructures
Ultrasound is among the most widely used biomedical imaging modalities, but has limited ability to image specific molecular targets due to the lack of suitable nanoscale contrast agents. Gas vesicles—genetically encoded protein nanostructures isolated from buoyant photosynthetic microbes—have recently been identified as nanoscale reporters for ultrasound. Their unique physical properties give gas vesicles significant advantages over conventional microbubble contrast agents, including nanoscale dimensions and inherent physical stability. Furthermore, as a genetically encoded material, gas vesicles present the possibility that the nanoscale mechanical, acoustic, and targeting properties of an imaging agent can be engineered at the level of its constituent proteins. Here, we demonstrate that genetic engineering of gas vesicles results in nanostructures with new mechanical, acoustic, surface, and functional properties to enable harmonic, multiplexed, and multimodal ultrasound imaging as well as cell-specific molecular targeting. These results establish a biomolecular platform for the engineering of acoustic nanomaterials.