DNA ‘lock and key’ allows for precision drug delivery to target cancer and other cells
January 12, 2016
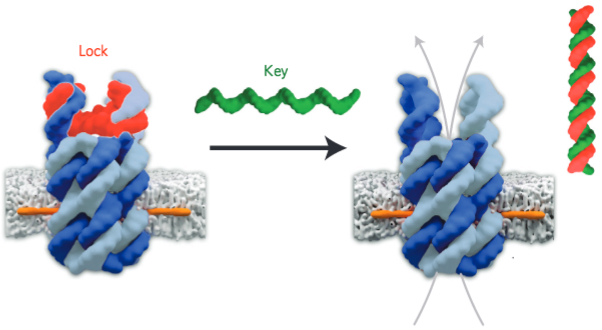
DNA-based lock-and-key pore design allows for precision delivery of drugs to cancer and other cells (credit: Stefan Howorka and Jonathan Burns/UCL)
Scientists at University College London (UCL) and Nanion Technologies in Munich have developed synthetic DNA-based pores that control which molecules can pass through a cell’s wall, achieving more precise drug delivery.
Therapeutics, including anti-cancer drugs, are ferried around the body in nanoscale carriers called vesicles, targeted to different tissues using biological markers. The new DNA-based pore design is intended to improve that process.
DNA Lock-and-key drug delivery
In operation, a drug-delivery vesicle (white in diagram above) carries the drug to a target cell for release.
The pore is designed as an open barrel made of six DNA-strand staves (blue and gray). The pore is kept closed by a DNA-strand “lock” (red) until the vesicle is prepared to release a drug. At that time, a “key” DNA-strand key (green) is released to hybridize (combine) with the lock DNA strand (forming the red-green helix on right), causing the pore to open and release the drug.*
The pore’s 2-nm-wide channel allows for selectively releasing small organic molecules, which include many medically important drug compounds.
This design for releasing drugs from vesicles improves on previous designs, in which drug release is triggered by temperature-induced leaky vesicle walls or with inserted peptide channels, both of which are less rigid and predictable than the new DNA mechanism.
The study was published Monday (Jan. 11) in Nature Nanotechnology. According to lead author Stefan Howorka, PhD., of UCL Chemistry, the researchers plan to test the synthetic pores in release of a variety of pharmaceutically active biomolecules, including anti-cancer drugs.
The research was funded by the Biotechnology and Biological Sciences Research Council (BBSRC), Leverhulme Trust and UCL Chemistry.
* Drugs are usually hydrophilic (water-loving), so pores are also designed to be hydrophilic, but that makes it difficult to attach pores to a vesicle membrane (which is hydrophobic, or fat-hating), so a hydrophobic cholesterol-based membrane (orange) was used to anchor the pore (blue and gray) to the membrane.
Abstract of A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane
Biological ion channels are molecular gatekeepers that control transport across cell membranes. Recreating the functional principle of such systems and extending it beyond physiological ionic cargo is both scientifically exciting and technologically relevant to sensing or drug release. However, fabricating synthetic channels with a predictable structure remains a significant challenge. Here, we use DNA as a building material to create an atomistically determined molecular valve that can control when and which cargo is transported across a bilayer. The valve, which is made from seven concatenated DNA strands, can bind a specific ligand and, in response, undergo a nanomechanical change to open up the membrane-spanning channel. It is also able to distinguish with high selectivity the transport of small organic molecules that differ by the presence of a positively or negatively charged group. The DNA device could be used for controlled drug release and the building of synthetic cell-like or logic ionic networks.