Eco-friendly ‘pre-fab’ self-assembling nanoparticles could revolutionize nano manufacturing
August 14, 2014
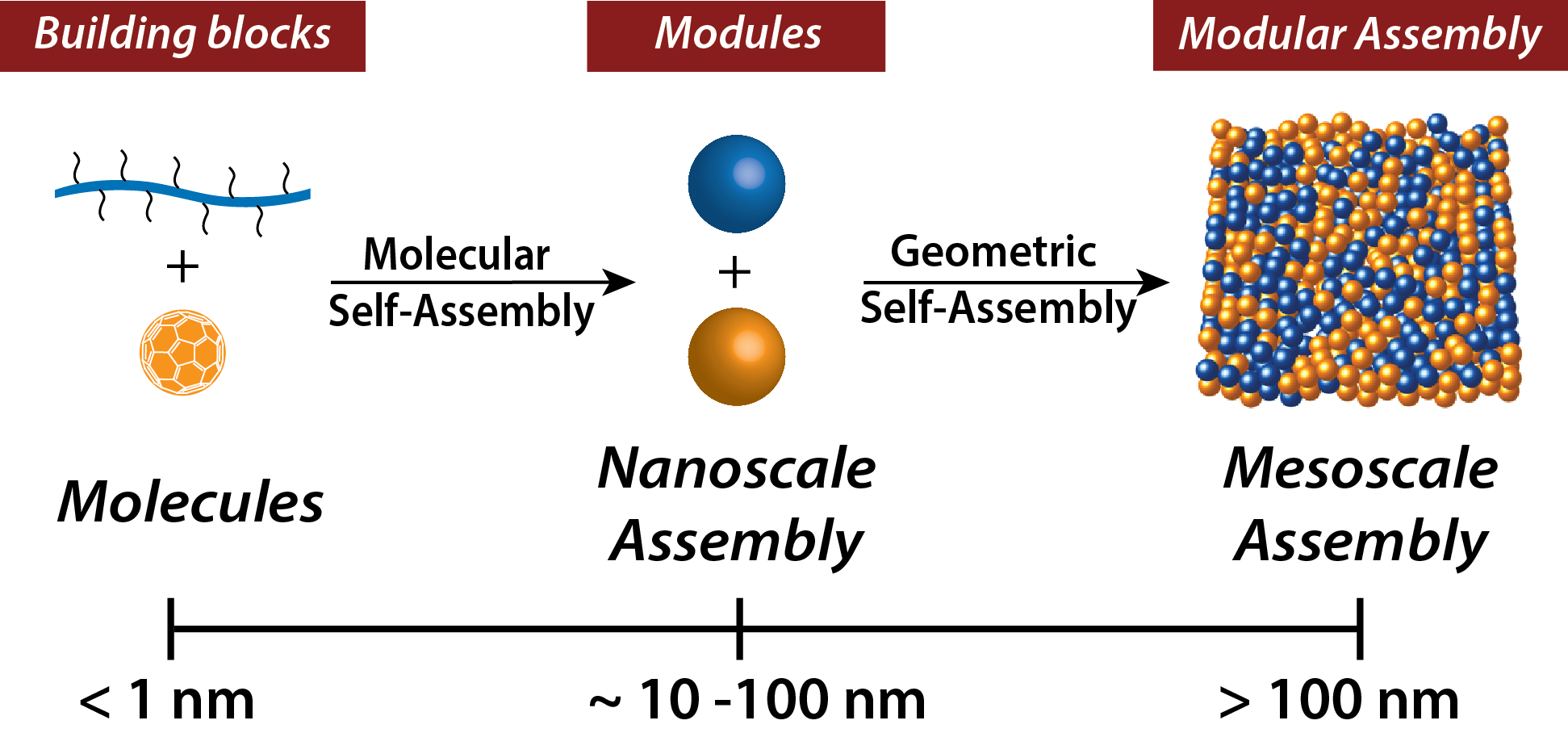
Pre-assembly of molecules and polymer chains from the molecular scale to form nanoscale “pre-fab” building modules will allow for making large, mesoscale (mid range between nano and macro) assemblies for devices. In this way, the mesoscale patterns can utilize the improved electronic properties in the pre-assembled modules. (Credit: University of Massachusetts Amherst)
University of Massachusetts Amherst scientists have developed a breakthrough technique for creating water-soluble nano-modules and controlling molecular assembly of nanoparticles over multiple length scales.
The new method should reduce the time nanotech manufacturing firms spend in trial-and-error searches for materials to make electronic devices such as solar cells, organic transistors, and organic light-emitting diodes.
“The old way can take years,” says materials chemist Paul Lahti, co-director with Thomas Russell of UMass Amherst’s Energy Frontiers Research Center (EFRC), supported by the U.S. Department of Energy.
“Another of our main objectives is to make something that can be scaled up from nano- to mesoscale, and our method does that. It is also much more ecologically friendly because we use water instead of dangerous solvents in the process.:
Details are in the current issue of Nano Letters.
“In this paper, we worked on glass, but we want to translate to flexible materials and produce roll-to-roll manufactured materials with water,” said chemist Dhandapani Venkataraman, lead investigator. “We expect to actually get much greater efficiency.” He suggests that reaching 5 percent power conversion efficiency would justify the investment for making small, flexible solar panels to power devices such as smart phones.
Energy and carbon dioxide savings
If the average smart phone uses 5 watts of power and all 307 million United States users switched from batteries to flexible solar, it could save more than 1500 megawatts per year, Venkataraman estimates. “That’s nearly the output of a nuclear power station,” he says, “and it’s more dramatic when you consider that coal-fired power plants generate 1 megawatt and release 2,250 lbs. of carbon dioxide. So if a fraction of the 6.6 billion mobile phone users globally changed to solar, it would reduce our carbon footprint a lot.”
Doctoral student and first author Tim Gehan says that organic solar cells made in this way can be semi-transparent, as well, “so you could replace tinted windows in a skyscraper and have them all producing electricity during the day when it’s needed. And processing is much cheaper and cleaner with our cells than in traditional methods.”
Scaling up to go commercial
“Consistently achieving the optimum morphology in organic photovoltaics from the molecular to mesoscopic and macroscopic length scales has been challenging, as it depends on the interplay and delicate balance of multiple kinetic processes,” Venkataraman explained in a KurzweilAI emailinterview with him and Lahti.
“Our innovation now provides a rational and consistent way to tailor and optimize each active-layer component assembly over multiple length scales. Moreover, we can use water instead of toxic haloarenes solvents for the fabrication the active layers. This is important because it will reduce the fabrication cost. Our innovation also now provides a general strategy for generating functional mesoscale assemblies while exercising control over molecular assembly at multiple length scales.
“A patent for this methodology is already pending. We expect to demonstrate that the method can be extended to a wide variety of organic materials over the coming 1–2 years. That will be important for commercial enterprises to feel comfortable enough to consider utilizing this process in place of existing technologies.”
“This is ground-breaking, especially to incorporate variable amounts of electroactive nanoparticles made of different substances,” added Lahti. “The possibilities are almost endless, in terms of different particle sizes and shapes, and of different nanoparticle mixtures made of different materials.”
This work at the Polymer-Based Materials for Harvesting Solar Energy is part of an EFRC supported by the U.S. DOE’s Office of Basic Energy Sciences.
University of Massachusetts Amherst | DV’s Research on solar cells.
Abstract of Nano Letters paper
We address here the need for a general strategy to control molecular assembly over multiple length scales. Efficient organic photovoltaics require an active layer comprised of a mesoscale interconnected networks of nanoscale aggregates of semiconductors. We demonstrate a method, using principles of molecular self-assembly and geometric packing, for controlled assembly of semiconductors at the nanoscale and mesoscale. Nanoparticles of poly(3-hexylthiophene) (P3HT) or [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) were fabricated with targeted sizes. Nanoparticles containing a blend of both P3HT and PCBM were also fabricated. The active layer morphology was tuned by the changing particle composition, particle radii, and the ratios of P3HT:PCBM particles. Photovoltaic devices were fabricated from these aqueous nanoparticle dispersions with comparable device performance to typical bulk-heterojunction devices. Our strategy opens a revolutionary pathway to study and tune the active layer morphology systematically while exercising control of the component assembly at multiple length scales.