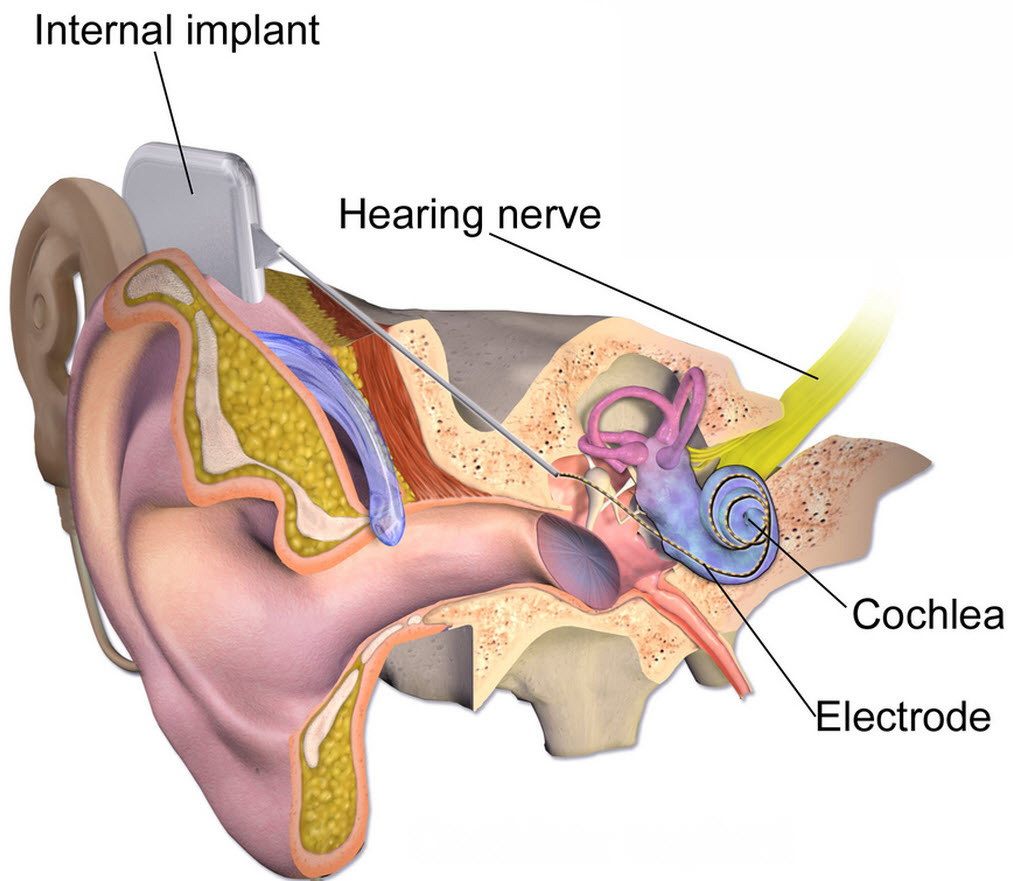
Electric pulses for delivering gene therapy may restore hearing quality for cochear-implant users
April 28, 2014
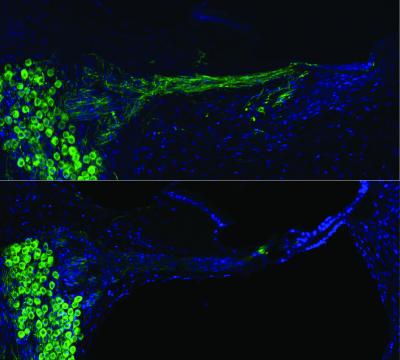
Regenerated auditory nerves after gene therapy (top) compared with no treatment (below) (credit: UNSW Translational Neuroscience Facility)
Researchers at UNSW Australia have used electrical pulses from a cochlear implant to deliver gene therapy in animals, successfully regrowing auditory nerves.
The research also heralds a possible new way of treating other neurological disorders, including Parkinson’s disease, and psychiatric conditions such as depression through this novel way of delivering gene therapy, the researchers say.
The research was published Thursday April 24 in the journal Science Translational Medicine.
“People with cochlear implants do well with understanding speech, but their perception of pitch can be poor, so they often miss out on the joy of music,” says UNSW Professor Gary Housley, senior author of the research paper and Director of the Translational Neuroscience Facility at UNSW Medicine.
The work centers on regenerating surviving nerves after age-related or environmental hearing loss, using existing cochlear technology. The therapy uses a few electric pulses administered during the implant procedure. The cochlear implants are then “surprisingly efficient” at localized gene therapy in the animal model.
“This research breakthrough is important because while we have had very good outcomes with our cochlear implants so far, if we can get the nerves to grow close to the electrodes and improve the connections between them, then we’ll be able to have even better outcomes in the future,” says Jim Patrick, Chief Scientist and Senior Vice-President, Cochlear Limited.
It has long been established that the auditory nerve endings regenerate if neurotrophins — a naturally occurring family of proteins crucial for the development, function and survival of neurons — are delivered to the auditory portion of the inner ear, the cochlea. But until now, research has stalled because safe, localized delivery of the neurotrophins can’t be achieved using either drug delivery or viral-based gene therapy.
Stimulating DNA delivery with electrical pulses
So Housley and his team at UNSW developed a way of using electrical pulses from the cochlear implant to stimulate delivery of the DNA to the cells close to the array of implanted electrodes. These cells then produce neurotrophins.
The neurotrophin production dropped away after a couple of months, but Housley says that ultimately, changes in the hearing nerve may be maintained by the natural ongoing neural activity generated by the cochlear implant.
“We think it’s possible that in the future this gene delivery would only add a few minutes to the implant procedure,” says the paper’s first author, Jeremy Pinyon, whose PhD is based on this work.
“The surgeon who installs the device would inject the DNA solution into the cochlea and then fire electrical impulses to trigger the DNA transfer once the implant is inserted.”
Integration of this technology into other “bionic” devices such as electrode arrays used in deep brain stimulation (for the treatment of Parkinson’s disease and depression, for example) could also provide opportunities for safe, directed gene therapy of complex neurological disorders, the researchers suggest.
“Our work has implications far beyond hearing disorders,” says co-author Associate Professor Matthias Klugmann, from the UNSW Translational Neuroscience Facility research team. “Gene therapy has been suggested as a treatment concept even for devastating neurological conditions and our technology provides a novel platform for safe and efficient gene transfer into tissues as delicate as the brain.”
The research is supported by Cochlear Limited through an Australian Research Council Linkage Project grant.
The next generation of bionic ear technology is a step closer to reality, with researchers at UNSW Australia using the cochlear implant itself to deliver DNA to stimulate the regrowth of auditory nerve cells. Professor Gary Housley is leading the development of the technique, which promises to significantly improve the hearing experience of people requiring a cochlear implant.
Animation showing how the cochlear implant delivers a DNA solution to stimulate regrowth of auditory nerve cells. A team at UNSW Australia lead by Professor Gary Housley is developing the technique with the aim of significantly improving the hearing experience of people requiring a cochlear implant.
Abstract of Science Translational Medicine paper
The cochlear implant is the most successful bionic prosthesis and has transformed the lives of people with profound hearing loss. However, the performance of the “bionic ear” is still largely constrained by the neural interface itself. Current spread inherent to broad monopolar stimulation of the spiral ganglion neuron somata obviates the intrinsic tonotopic mapping of the cochlear nerve. We show in the guinea pig that neurotrophin gene therapy integrated into the cochlear implant improves its performance by stimulating spiral ganglion neurite regeneration. We used the cochlear implant electrode array for novel “close-field” electroporation to transduce mesenchymal cells lining the cochlear perilymphatic canals with a naked complementary DNA gene construct driving expression of brain-derived neurotrophic factor (BDNF) and a green fluorescent protein (GFP) reporter. The focusing of electric fields by particular cochlear implant electrode configurations led to surprisingly efficient gene delivery to adjacent mesenchymal cells. The resulting BDNF expression stimulated regeneration of spiral ganglion neurites, which had atrophied 2 weeks after ototoxic treatment, in a bilateral sensorineural deafness model. In this model, delivery of a control GFP-only vector failed to restore neuron structure, with atrophied neurons indistinguishable from unimplanted cochleae. With BDNF therapy, the regenerated spiral ganglion neurites extended close to the cochlear implant electrodes, with localized ectopic branching. This neural remodeling enabled bipolar stimulation via the cochlear implant array, with low stimulus thresholds and expanded dynamic range of the cochlear nerve, determined via electrically evoked auditory brainstem responses. This development may broadly improve neural interfaces and extend molecular medicine applications.