Engineered biomaterial may regenerate damaged skeletal muscle
August 1, 2014

Jeffrey Wolchok, right, works with a biomaterial that can regenerate damaged skeletal muscle (credit: University of Arkansas)
A biomaterial that can regenerate damaged skeletal muscle is being developed by University of Arkansas biomedical engineering researcher Jeffrey Wolchok, funded by a National Institutes of Health three-year, $437,248 grant.
Living cells secrete fibrous proteins and polysaccharide gels called extracellular matrix, which support cell survival and tissue strength. Minor muscle injuries affect tissue cells but not the extracellular components. In severe injuries, however, the extracellular matrix does not function properly and cannot initiate the healing process.
Engineered “muscle-mimics” provide the molecules necessary to cue regeneration.
“When significant muscle volume is lost, the physical and chemical cues provided by the extracellular matrix are missing, and the volumetric muscle loss is instead replaced with scar tissue,” said Jeffrey Wolchok, assistant professor of biomedical engineering.

The sacrificial scaffold + ECM before the sacrificial scaffold is dissolved (credit: University of Arkansas)
Building muscle-mimicking biomaterials
The grant will allow Wolchok and his research team to build muscle-mimicking biomaterials using a cellular approach and to test the material’s regenerative capacities. “Like collecting honey from bees, we collect the extracellular matrix secreted by cells and put it together to build a biomaterial,” Wolchok said.
Wolchok grows cells inside a porous foam. These cells secrete extracellular matrix into the pores of the foam. The foam can then be sacrificed (dissolved in a solvent) so only the extracellular matrix remains.
“We can use the sacrificial foam technique to build an engineered muscle-mimic scaffold capable of regenerating damaged skeletal muscle,” Wolchok said.
One example where this regeneration would help is rotator cuff injuries. Each year physicians operate on 400,000 rotator cuff injuries, and approximately one of three of those patients will repeat the surgery because the tendon holding the muscles together re-ruptures.
Fatty tissue degeneration following the initial injury contributes to post-surgery tendon tears. Surgeons would use the muscle-mimicking biomaterial to repair the damaged skeletal muscle, including the rotator cuff muscles.
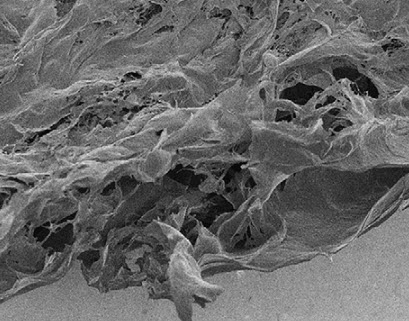
SEM of cell-derived extracellular matrix (credit: Jeffrey C. Wolchok and Patrick A. Tresco/Biomaterials)
Wolchok said his engineered muscle-mimic could be injected into the injured tissue directly or combined with stem cells harvested from the patient. Potentially, the stem cells and the extracellular matrix in the mimic would communicate with native cells and stimulate the regeneration of healthy muscle.
“Even a modest reduction to the failure rate could eliminate tens of thousands of repeat procedures and greatly reduce rotator cuff-related health care expenses,” Wolchok said.
“The current standard of care to replace lost muscle is the transfer of autologous muscle flaps,” Wolchok explained to KurzweilAI in an email interview. “Flap transfers have been used to reconstruct forearm, elbow, and shoulder muscles. Yet, the transfer of muscle tissue is an invasive procedure that causes significant donor site morbidity.
“We suggest that a readily available ‘off the shelf’ biomaterial that provides the appropriate regenerative cues by mimicking the properties of decellularized skeletal muscle (DSM) is an attractive alternative.
However, he said the current approach to create ECM materials is top-down: start with whole tissue (animal or human), strip away the cells to produce a scaffold consisting of the remaining ECM. “Our approach is bottom-up: start with the cells, collect the ECM that they produce and use that ECM to build a scaffold.
“This project will take 3–5 years. If it proves effective, it would be at least another 5+ years (optimistically) to get to clinical studies; we are still at the high-risk early stages.”
Wolchok is the primary investigator in the Regenerative Biomaterials Laboratory at the University of Arkansas. He studies biomaterials derived from cells, tissue engineering and regenerative medicine and bioreactors. He also investigates the design of medical devices and the influence of mechanical force on cell behavior.
Abstract of Biomaterials paper
Extracellular matrix derived from human and animal tissues is being used to repair and reconstruct a variety of tissues clinically. The utility of such constructs is limited by the geometry, composition and constitutive properties of the tissue or organ from which the ECM is harvested. To address this limitation, we have developed an approach to isolate extracellular matrix in bulk from populations of living cells grown in culture on three-dimensional substrates. Human biopsy derived fibroblasts were seeded within open-cell foams and cultured in-vitro for periods up to three weeks, after which the synthetic component was removed by incubation in a water miscible solvent. After several wash steps and lyophilization, a white, lacy, multi-molecular construct was isolated. Tandem mass spectroscopy showed that it contained 22 extracellular matrix constituents, including such proteins and proteoglycans as collagen type I and type III, fibronectin, transforming growth factor beta, decorin and biglycan among others. On average 47 mg of construct was isolated for each gram of synthetic substrate initially seeded with cells. The biomaterial harvested from human tracheal fibroblasts had an elastic modulus (250 kPa) and a composition similar to that of human vocal fold tissue, and supported reseeding with human tracheal derived fibroblasts. An important finding was that the approach was useful in isolating ECM from a variety of cell lineages and developmental stages including skin fibroblasts, brain derived astrocytes and mesenchymal stem cells. The results, together with the archival literature, suggest that the approach can be used to produce a range of cell derived constructs with unique physical and chemical attributes for a variety of research and medical applications.