Exploring long-range communications in the brain
March 23, 2016
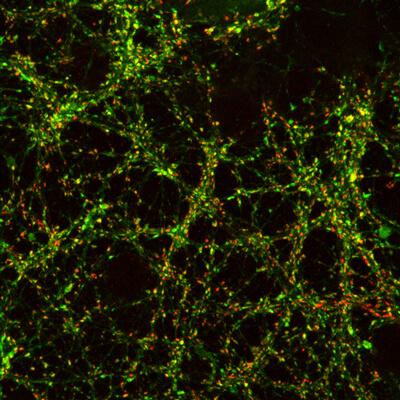
Red and green dots reveal a region in the brain that that is very dense with synapses. A optically activated fluorescent protein allows Ofer Yizhar, PhD, and his group to record the activity of the synapses. (credit: Weizmann Institute of Science)
Weizmann Institute of Science researchers have devised a new way to track long-distance communications between nerve cells in different areas of the brain. They used optogenetic techniques (using genetic engineering of neurons and laser light in thin optical fibers to temporarily silence long-range axons, effectively leading to a sustained “disconnect” between two distant brain nodes.
By observing what happens when crucial connections are disabled, the researchers could begin to determine the axons’ role in the brain. Mental and neurological diseases are often thought to result from changes in long-range brain connectivity, so these studies could contribute to a better understanding of the mechanisms behind health and disease in the brain.
The study, published in Nature Neuroscience, “led us to a deeper understanding of the unique properties of the axons and synapses that form the connections between neurons,” said Ofer Yizhar, PhD, in the Weizmann Institute of Science’s Neurobiology Department. “We were able to uncover the responses of axons to various optogenetic manipulations. Understanding these differences will be crucial to unraveling the mechanisms for long-distance communication in the brain.”
Abstract of Biophysical constraints of optogenetic inhibition at presynaptic terminals
We investigated the efficacy of optogenetic inhibition at presynaptic terminals using halorhodopsin, archaerhodopsin and chloride-conducting channelrhodopsins. Precisely timed activation of both archaerhodopsin and halorhodpsin at presynaptic terminals attenuated evoked release. However, sustained archaerhodopsin activation was paradoxically associated with increased spontaneous release. Activation of chloride-conducting channelrhodopsins triggered neurotransmitter release upon light onset. Thus, the biophysical properties of presynaptic terminals dictate unique boundary conditions for optogenetic manipulation.