Gene therapy for aging-associated decline
May 16, 2012
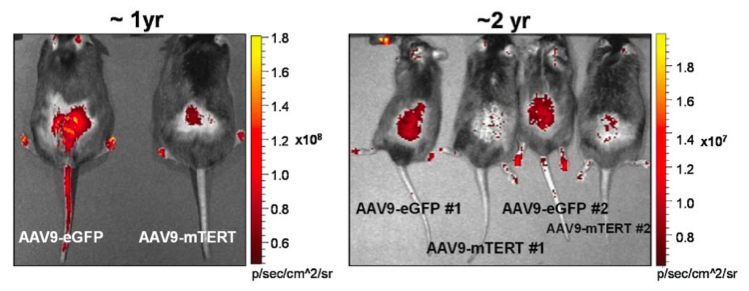
Direct GFP fluorescence in shaved back skin of mice treated with the indicated vectors (credit: Bruno Bernardes de Jesus et al./EMBO Molecular Medicine)
Mouse lifespan was extended up to 24 percent with a single gene treatment in research at the Spanish National Cancer Research Centre (CNIO), using gene therapy, a strategy never before employed to combat aging.
Mice treated at the age of one lived longer by 24% on average, and those treated at the age of two, by 13%. The therapy, furthermore, produced an appreciable improvement in the animals’ health, delaying the onset of age-related diseases – like osteoporosis and insulin resistance – and achieving improved readings on ageing indicators like neuromuscular coordination.
The gene therapy utilised consisted of treating the animals with a DNA-modified virus, the viral genes having been replaced by those of the telomerase enzyme, with a key role in aging. Telomerase repairs the extremes of chromosomes, known as telomeres, and in doing so slows the cell’s and therefore the body’s biological clock. When the animal is infected, the virus acts as a vehicle depositing the telomerase gene in the cells.
This study “shows that it is possible to develop a telomerase-based anti-aging gene therapy without increasing the incidence of cancer,” the authors affirm. “Aged organisms accumulate damage in their DNA due to telomere shortening, [this study] finds that a gene therapy based on telomerase production can repair or delay this kind of damage.”
‘Resetting’ the biological clock
Telomeres are the caps that protect the end of chromosomes, but they cannot do so indefinitely: each time the cell divides the telomeres get shorter, until they are so short that they lose all functionality. The cell, as a result, stops dividing and ages or dies. Telomerase gets round this by preventing telomeres from shortening or even rebuilding them. What it does, in essence, is stop or reset the cell’s biological clock.
But in most cells the telomerase gene is only active before birth; the cells of an adult organism, with few exceptions, have no telomerase. The exceptions in question are adult stem cells and cancer cells, which divide limitlessly and are therefore immortal – in fact several studies have shown that telomerase expression is the key to the immortality of tumor cells.
It is precisely this risk of promoting tumor development that has set back the investigation of telomerase-based anti-ageing therapies.
In 2007, CNIO scientists proved that it was feasible to prolong the lives of transgenic mice whose genome had been permanently altered at the embryonic stage, by causing their cells to express telomerase and, also, extra copies of cancer-resistant genes. These animals live 40% longer than is normal and do not develop cancer.
The mice subjected to the gene therapy now under test are likewise free of cancer. Researchers believe this is because the therapy begins when the animals are adult so do not have time to accumulate sufficient number of aberrant divisions for tumours to appear.
Also important is the kind of virus employed to carry the telomerase gene to the cells. The authors selected demonstrably safe viruses that have been successfully used in gene therapy treatment of haemophilia and eye disease. Specifically, they are non-replicating viruses derived from others that are non-pathogenic in humans.
Although this therapy may not find application as an anti-aging treatment in humans, in the short term at least, it could open up a new treatment option for ailments linked with the presence in tissue of abnormally short telomeres, as in some cases of human pulmonary fibrosis.
The vector the scientists use expresses the target gene (telomerase) over a long period, so they were able to apply a single treatment. This might be the only practical solution for an anti-ageing therapy, since other strategies would require the drug to be administered over the patient’s lifetime, multiplying the risk of adverse effects.
Bruno Bernardes de Jesus et al., Telomerase gene therapy in adult and old mice delays ageing and increases longevity without increasing cancer, EMBO Molecular Medicine, 2012 DOI: 10.1002/emmm.201200245