Genome-wide search reveals >750 worm genes involved in long-term memory
January 25, 2015
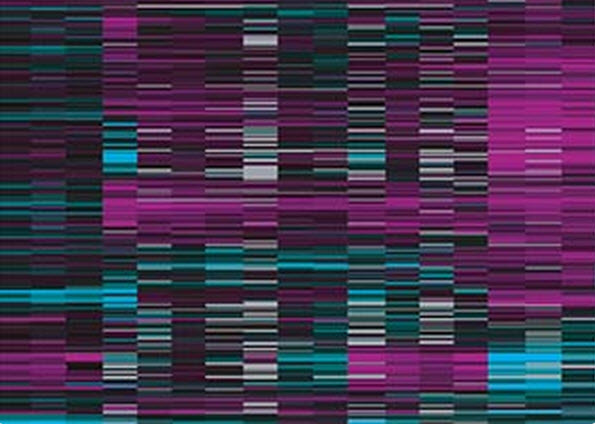
Whole-genome expression data reveals new genes involved in long-term memory formation in worms (credit: Murphy lab)
A new Princeton University study has identified more than 750 genes involved in long-term memory in the worm — part of research aimed at finding ways to retain cognitive abilities during aging, including compounds.
The study takes a different approach than the recent ENIGMA study, which identified genetic mutations in humans related to brain aging.
The new study, published in the journal Neuron, included many genes that had not been found previously and that could serve as targets for future research, said senior author Coleen Murphy, an associate professor of molecular biology at Princeton and the Lewis-Sigler Institute for Integrative Genomics.
A molecule that turns on long-term-memory genes
The researchers pinpointed* genes that are “turned on” by a molecule known as CREB (cAMP-response element-binding protein), known to be required for long-term memory in many organisms, including worms and mice.
“There is a pretty direct relationship between CREB and long-term memory,” Murphy said, “and many organisms lose CREB as they age.” By studying the CREB-activated genes involved in long-term memory, the researchers hope to better understand why some organisms lose their long-term memories as they age.
Worms are a perfect system in which to explore that question, Murphy said. The worm Caenorhabditis elegans has only 302 neurons, whereas a typical mammalian brain contains billions of the cells.
“Worms use the same molecular machinery that higher organisms, including mammals, use to carry out long-term memory,” said Murphy. “We hope that other researchers will take our list and look at the genes to see whether they are important in more complex organisms.”
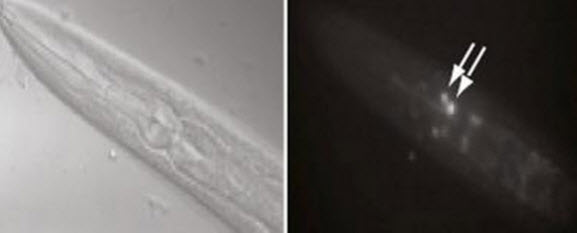
Long-term memory training in worms (left) led to induction of the transcription factor CREB in AIM neurons (shown by arrows in right). CREB-induced genes were shown to be involved in forming long-term memories in worm neurons. (Credit: Murphy lab)
The next step, said Murphy, is to find out what these newly recognized long-term memory genes do when they are activated by CREB. For example, the activated genes may strengthen connections between neurons.
* To identify the genes, the researchers first instilled long-term memories in the worms by training them to associate meal-time with a butterscotch smell. Trained worms were able to remember that the butterscotch smell means dinner for about 16 hours, a significant amount of time for the worm.
The researchers then scanned the genomes of both trained worms and non-trained worms, looking for genes turned on by CREB. The researchers detected 757 CREB-activated genes in the long-term memory-trained worms, and showed that these genes were turned on primarily in worm cells called the AIM interneurons.
They also found CREB-activated genes in non-trained worms, but the genes were not turned on in AIM interneurons and were not involved in long-term memory. CREB turns on genes involved in other biological functions such as growth, immune response, and metabolism. Throughout the worm, the researchers noted distinct non-memory (or “basal”) genes in addition to the memory-related genes.
Abstract of Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs
Induced CREB activity is a hallmark of long-term memory, but the full repertoire of CREB transcriptional targets required specifically for memory is not known in any system. To obtain a more complete picture of the mechanisms involved in memory, we combined memory training with genome-wide transcriptional analysis of C. elegans CREB mutants. This approach identified 757 significant CREB/memory-induced targets and confirmed the involvement of known memory genes from other organisms, but also suggested new mechanisms and novel components that may be conserved through mammals. CREB mediates distinct basal and memory transcriptional programs at least partially through spatial restriction of CREB activity: basal targets are regulated primarily in nonneuronal tissues, while memory targets are enriched for neuronal expression, emanating from CREB activity in AIM neurons. This suite of novel memory-associated genes will provide a platform for the discovery of orthologous mammalian long-term memory components.