Graphene walls could allow for chips with 100 trillion FETs per sq. centimeter
October 3, 2011
Tiny strips of graphene — one-atom-thick sheets of carbon — can stand tall on a substrate, scientists at Rice University and Hong Kong Polytechnic University have found. This means arrays of graphene walls could become ultrahigh-density components of electronic or spintronic devices, significantly extending chip density — eventually, even sub-nanometer devices.
Calculations by Rice theoretical physicist Boris Yakobson, Assistant Professor Feng Ding of Hong Kong Polytechnic, and their collaborators showed substrates of diamond (and also nickel) could chemically bind the edge of a strip of a graphene nanoribbon. Because the contact is so slight, the graphene walls retain nearly all of their inherent electrical or magnetic properties.
100 trillion FETs per sq. centimeter
And because they’re so thin, the scientists calculated a theoretical potential of putting 100 trillion graphene-wall field-effect transistors (FETs) on a square-centimeter chip.
That potential alone may make it possible to blow past the limits implied by Moore’s Law — something Yakobson once discussed with Intel founder Gordon Moore himself. “We met in Montreal, when nano was a new kid on the block, and had a good conversation. Moore liked to talk about silicon wafers in terms of real estate. Following his metaphor, an upright architecture would increase the density of circuits on a chip — like going from ranch-style houses in Texas to skyscraper condos in Hong Kong. This kind of strategy may help sustain Moore’s Law for an extra decade.”
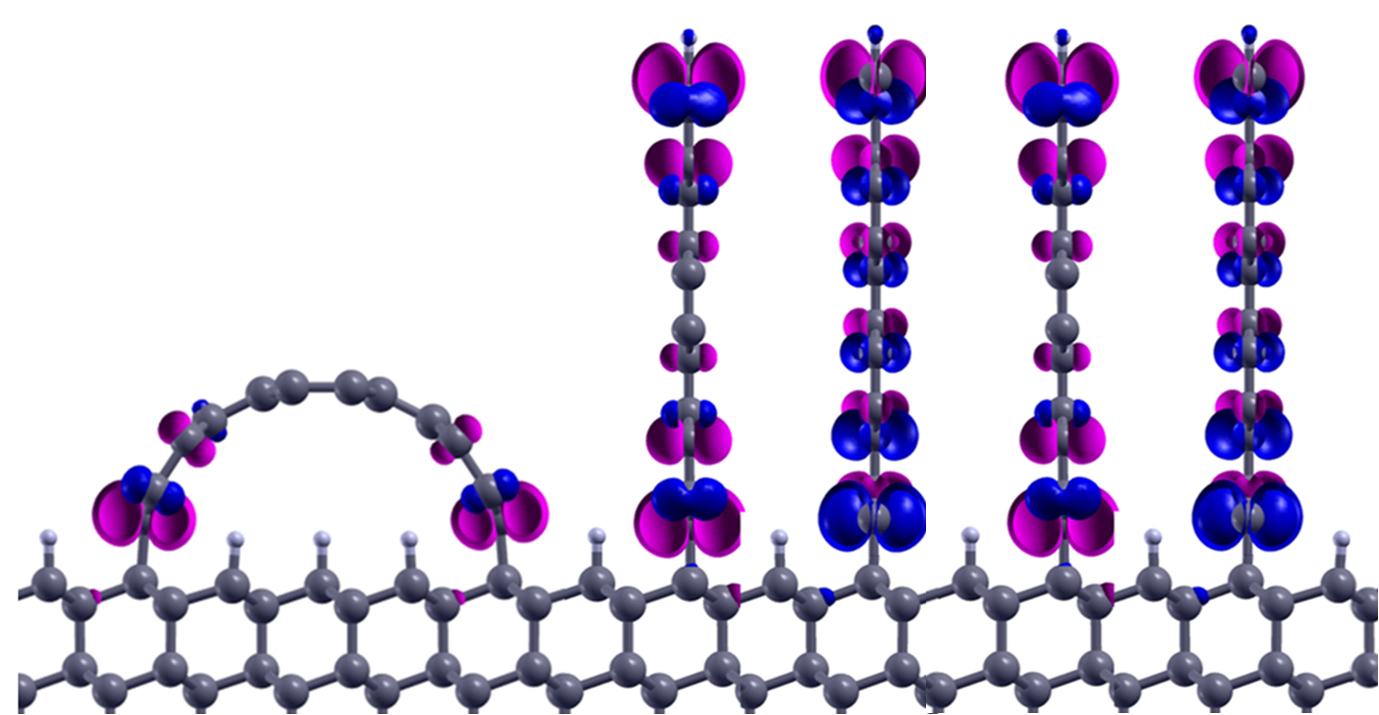
Researchers at Rice University and Hong Kong Polytechnic University calculated that graphene nanoribbons (blue and purple) could stand up on diamond or nickel, or even form arches. Up to 100 trillion of such graphene walls could fit on a square-centimeter chip. (Credit: Feng Ding/Hong Kong Polytechnic University)
A sheet of material a fraction of a nanometer wide is pretty pliable, he said, but the laws of physics are on its side: binding energies between carbon in the diamond matrix and carbon in graphene are maximized at the edge, and the molecules bind strongly at a 90-degree angle. Minimal energy is required for the graphene to stand upright, which is its preferred state. (Walls on a nickel substrate would be angled at about 30 degrees, the researchers found.)
Yakobson said the walls could be as close to each other as .7 nm (7/10 of a nanometer), which would maintain the independent electronic properties of individual nanoribbons. They could potentially be grown on silicon, silicon dioxide, aluminum oxide, or silicon carbide.
Tuning graphene properties by changing their form
The research illustrated differences between walls made of two distinct types of graphene: zigzag and armchair, so-called because of the way their edges are shaped.
Sheets of graphene are considered semimetals that have limited use in electronics, because electrical current shoots straight through without resistance. However, armchair nanoribbons can become semiconductors; the thinner the ribbon, the larger the band gap, which is essential for transistors.
Zigzag nanoribbons, on the other hand, are magnetic. Electrons at their opposing edges spin in opposite directions, a characteristic that can be controlled by an electric current; this makes them suitable for spintronic devices.
In both cases, the electronic properties of the walls can be tuned by changing their height.
The researchers also suggested nanowalls could become nanoarches — by attaching opposing ends of a graphene ribbon to the substrate. Rather than lie flat on the diamond or nickel surface, the energies along the binding edges would naturally force the graphene strip to rise in the middle. It would essentially become a half-nanotube with its own set of potentially useful properties.
Precisely how to turn these two-dimensional building blocks into a three-dimensional device presents challenges, but the payoff is great, Yakobson said.
Ref.: Qinghong Yuan et.al., Upright Standing Graphene Formation on Substrates, J. Am. Chem. Soc., September 2011 [DOI: 10.1021/ja2037854]
Videos on Upright Standing Graphene Formation on Substrates
