‘Holy grail’ of breast-cancer prevention in high-risk women may be in sight
June 21, 2016
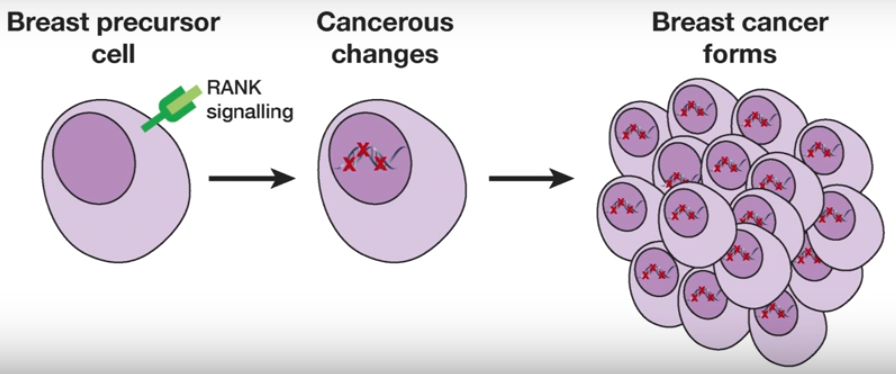
Breast cancer formation (credit: Walter and Eliza Hall Institute)
Australian researchers have discovered that an existing medication could have promise in preventing breast cancer in women carrying a faulty BRCA1 gene, who are at high risk of developing aggressive breast cancer.
Currently, many women with this mutation choose surgical removal of breast tissue and ovaries to reduce their chance of developing breast and ovarian cancer. Notably, in May 2013, actress Angelina Jolie, who reportedly had with an estimated 87 per cent risk of breast cancer and 50 per cent risk of ovarian cancer, chose to have d2ouble mastectomy with breast reconstruction.
Women with mutation have an approximately 65% cumulative risk of developing breast cancer by age 70, the researchers note, based on a 2003 combined analysis of 22 studies.
A drug option
But now, another option may be be possible, as 16 scientists (most in Australia) report in an advance online paper in Nature Medicine this week.
The researchers discovered that pre-cancerous cells could be identified by a marker protein called RANK. A concurrent study led by an Austrian group had also identified the importance of RANK.
This was an important breakthrough, they said, because an inhibitor of the RANK signalling pathway was already in clinical use: the drug denosumab. The researchers suggest the drug may have potential to prevent breast cancer from developing.
If confirmed in clinical studies, this would provide a non-surgical option to prevent breast cancer in women with elevated genetic risk.
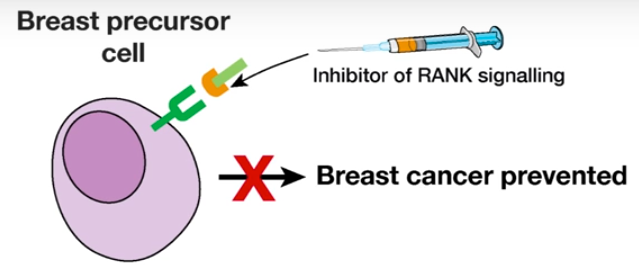
Breast cancer prevention (credit: Walter and Eliza Hall Institute)
“This is potentially a very important discovery for women who carry a faulty BRCA1 gene, who have few other options,” said Walter and Eliza Hall Institute of Medical Research professor Geoffrey J. Lindeman. “Current cancer prevention strategies for these women include surgical removal of the breasts and/or ovaries, which can have serious impacts on people’s lives.
“To progress this work, denosumab would need to be formally tested in clinical trials in this setting as it is not approved for breast cancer prevention,” he said.
The research was published this week in Nature Medicine and was supported by The National Breast Cancer Foundation, The Qualtrough Cancer Research Fund, The Joan Marshall Breast Cancer Research Fund, the Australian Cancer Research Foundation, Cancer Council Victoria, the Cancer Therapeutics Cooperative Research Centre, an Amgen Preclinical Research Program Grant, the National Health and Medical Research Council, the Victorian Cancer Agency, and the Victorian Government Operational Infrastructure Support Scheme.
The Walter and Eliza Hall Institute | ‘Holy grail’ of breast cancer prevention in high-risk women may be in sight
Abstract of RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers
Individuals who have mutations in the breast-cancer-susceptibility gene BRCA1 (hereafter referred to as BRCA1-mutation carriers) frequently undergo prophylactic mastectomy to minimize their risk of breast cancer. The identification of an effective prevention therapy therefore remains a ‘holy grail’ for the field. Precancerous BRCA1mut/+ tissue harbors an aberrant population of luminal progenitor cells, and deregulated progesterone signaling has been implicated in BRCA1-associated oncogenesis. Coupled with the findings that tumor necrosis factor superfamily member 11 (TNFSF11; also known as RANKL) is a key paracrine effector of progesterone signaling and that RANKL and its receptor TNFRSF11A (also known as RANK) contribute to mammary tumorigenesis, we investigated a role for this pathway in the pre-neoplastic phase of BRCA1-mutation carriers. We identified two subsets of luminal progenitors (RANK+ and RANK−) in histologically normal tissue of BRCA1-mutation carriers and showed that RANK+ cells are highly proliferative, have grossly aberrant DNA repair and bear a molecular signature similar to that of basal-like breast cancer. These data suggest that RANK+ and not RANK− progenitors are a key target population in these women. Inhibition of RANKL signaling by treatment with denosumab in three-dimensional breast organoids derived from pre-neoplasticBRCA1mut/+ tissue attenuated progesterone-induced proliferation. Notably, proliferation was markedly reduced in breast biopsies from BRCA1-mutation carriers who were treated with denosumab. Furthermore, inhibition of RANKL in a Brca1-deficient mouse model substantially curtailed mammary tumorigenesis. Taken together, these findings identify a targetable pathway in a putative cell-of-origin population in BRCA1-mutation carriers and implicate RANKL blockade as a promising strategy in the prevention of breast cancer.