How aging cripples the immune system
August 7, 2015
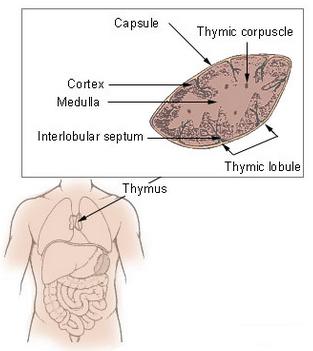
Thymus (credit: Wikimedia Commons)
Aging cripples the production of new immune cells, decreasing the immune system’s response to vaccines and putting the elderly at risk of infection, but antioxidants in the diet may slow this damaging process.
That’s a new finding by scientists from the Florida campus of The Scripps Research Institute (TSRI), published in an open-access paper in the journal Cell Reports.
The problem is focused on an organ called the thymus, which produces T lymphocytes (a type of white blood cell) — critical immune cells that must be continuously replenished so they can respond to new infections.
“The thymus begins to atrophy rapidly in very early adulthood, simultaneously losing its function,” said TSRI Professor Howard Petrie.
“This new study shows for the first time a mechanism for the long-suspected connection between normal immune function and antioxidants.”
How antioxidant enzyme deficiency leads to metabolic damage
Scientists have been hampered in their efforts to develop specific immune therapies for the elderly by a lack of knowledge of the underlying mechanisms of this process.
To explore these mechanisms, Petrie and his team developed a computational approach for analyzing the activity of genes in two major cell types in the thymus — stromal cells and lymphoid cells — in mouse tissues, which are similar to human tissues in terms of function and age-related atrophy.
The team found that stromal cells were specifically deficient in an antioxidant enzyme called catalase. That resulted in elevated levels of the reactive oxygen byproducts of metabolism, which cause accelerated metabolic damage.*
New support for the “free-radical theory” of aging
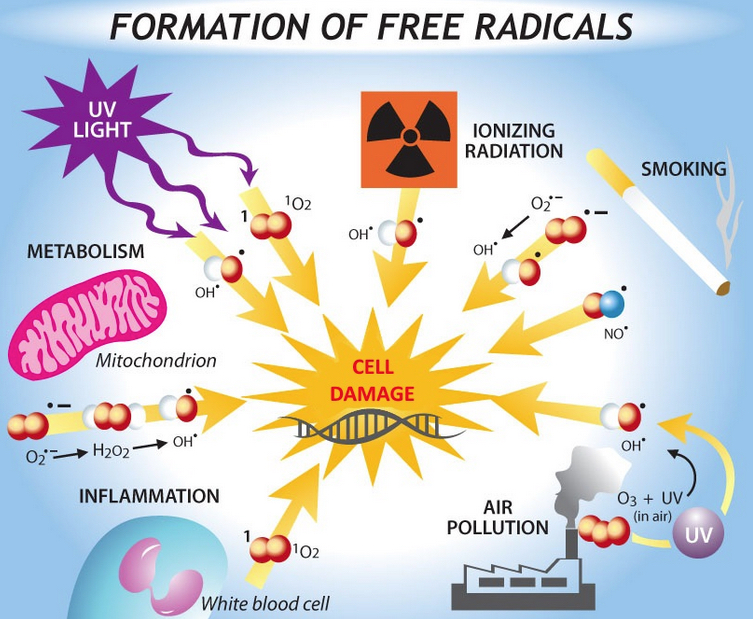
(Credit: Bethany Christmann)
Taken together, the findings provide support for the “free-radical theory” of aging, which proposes that reactive oxygen species (such as hydrogen peroxide), produced during normal metabolism (and from other sources) cause cellular damage that contributes to aging and age-related diseases.
Free radicals are especially reactive atoms or groups of atoms that have one or more unpaired electrons. Besides those produced in the body as a by-product of normal metabolism, they can also be introduced from an outside source, such as tobacco smoke or other toxins.
Other studies have suggested that sex hormones, particularly androgens such as testosterone, play a major role in the aging process. But according to the researchers, those studies have failed to answer the key question: why does the thymus atrophy so much more rapidly than other body tissues?
“There’s no question that the thymus is remarkably responsive to androgens,” Petrie noted, “but our study shows that the fundamental mechanism of aging in the thymus, namely accumulated metabolic damage, is the same as in other body tissues. However, the process is accelerated in the thymus by a deficiency in the essential protective effects of catalase, which is found at higher levels in almost all other body tissues.”
It’s complicated
However, lowering free radicals with antioxidants has not always conferred the expected benefits, according to Senior scientist and Buck Institute professor Judith Campisi, PhD.
In a study published August 3 online in Proceedings of the National Academy of Sciences, scientists in her lab bred mice that produced excess free radicals that damaged the mitochondria in their skin. Based on the free-radical theory, the scientists expected to see accelerated aging across the mouse lifespan.
Instead, they saw a surprising benefit in young animals: accelerated wound healing due to increased epidermal (skin) differentiation and re-epithelialization.
However, the mice paid a price over time. Campisi said mitochondrial damage from excess free radicals caused some of the skin cells to go into senescence — they stopped dividing and started accumulating. Campisi said that over time, the energy available to the epidermal stems cells was depleted — the stem cells simply became too scarce and the mice showed expected signs of aging: thin skin and poor wound healing.
“It may be that nature used free radicals to optimize skin health, but because this process is not deleterious to the organism until later in life, past its reproductive age, there was no [evolutionary benefit from evolving] ways to alter this mechanism,” suggested Michael Velarde, PhD, a postdoctoral fellow in the Campisi lab.
* To confirm the central role of catalase, the scientists increased levels of this enzyme in genetically altered animal models, resulting in preservation of thymus size for a much longer period. In addition, animals that were given two common dietary antioxidants, including vitamin C, were also protected from the effects of aging on the thymus.
Abstract of Metabolic Damage and Premature Thymus Aging Caused by Stromal Catalase Deficiency
T lymphocytes are essential mediators of immunity that are produced by the thymus in proportion to its size. The thymus atrophies rapidly with age, resulting in progressive diminution of new T cell production. This decreased output is compensated by duplication of existing T cells, but it results in gradual dominance by memory T cells and decreased ability to respond to new pathogens or vaccines. Here, we show that accelerated and irreversible thymic atrophy results from stromal deficiency in the reducing enzyme catalase, leading to increased damage by hydrogen peroxide generated by aerobic metabolism. Genetic complementation of catalase in stromal cells diminished atrophy, as did chemical antioxidants, thus providing a mechanistic link between antioxidants, metabolism, and normal immune function. We propose that irreversible thymic atrophy represents a conventional aging process that is accelerated by stromal catalase deficiency in the context of an intensely anabolic (lymphoid) environment.
Abstract of Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells
Tissue homeostasis declines with age partly because stem/progenitor cells fail to self-renew or differentiate. Because mitochondrial damage can accelerate aging, we tested the hypothesis that mitochondrial dysfunction impairs stem cell renewal or function. We developed a mouse model, Tg(KRT14-cre/Esr1)20Efu/J × Sod2tm1Smel, that generates mitochondrial oxidative stress in keratin 14-expressing epidermal stem/progenitor cells in a temporally controlled manner owing to deletion of Sod2, a nuclear gene that encodes the mitochondrial antioxidant enzyme superoxide dismutase 2 (Sod2). Epidermal Sod2 loss induced cellular senescence, which irreversibly arrested proliferation in a fraction of keratinocytes. Surprisingly, in young mice, Sod2 deficiency accelerated wound closure, increasing epidermal differentiation and reepithelialization, despite the reduced proliferation. In contrast, at older ages, Sod2 deficiency delayed wound closure and reduced epidermal thickness, accompanied by epidermal stem cell exhaustion. In young mice, Sod2 deficiency accelerated epidermal thinning in response to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate, phenocopying the reduced regeneration of older Sod2-deficient skin. Our results show a surprising beneficial effect of mitochondrial dysfunction at young ages, provide a potential mechanism for the decline in epidermal regeneration at older ages, and identify a previously unidentified age-dependent role for mitochondria in skin quality and wound closure.