How lizards regenerate their tails: researchers discover genetic ‘recipe’
August 22, 2014
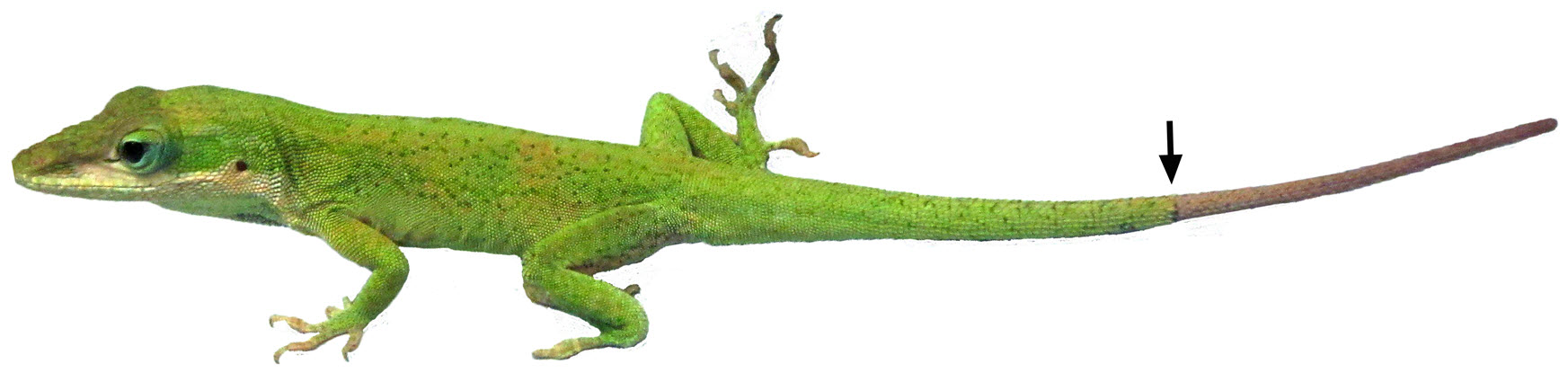
Researchers have discovered the genetic “recipe” for how the green anole lizard (Anolis carolinensis) can grow back a lost tail (credit: Hutchins et al./PLoS ONE)
Arizona State University scientists have discovered the genetic “recipe” for lizard tail regeneration, which may help develop future therapies for spinal cord injuries.
The team studied the regenerating tail of the green anole lizard (Anolis carolinensis), which when caught by a predator, can lose its tail and then grow it back.
The findings were published Aug. 20 in the journal PLOS ONE (open access).
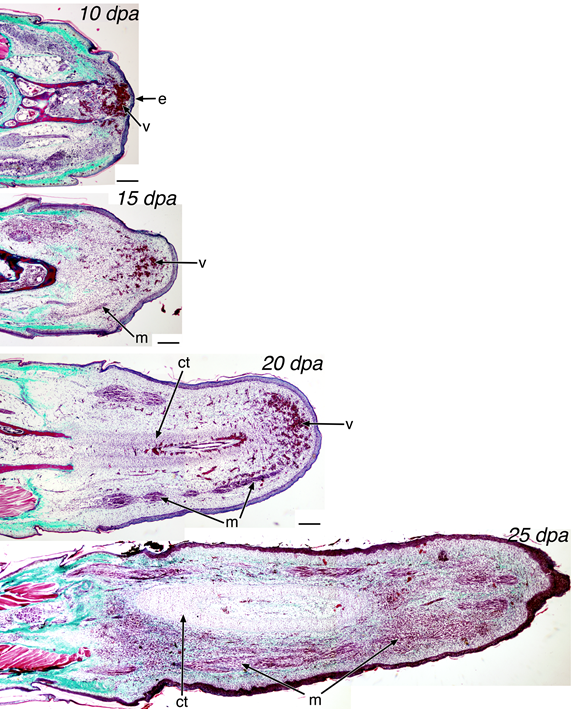
The stages of lizard tail regeneration (credit: Hutchins et al./PLoS ONE)
The genetic regeneration pathway
“Lizards basically share the same toolbox of genes as humans,” said lead author Kenro Kusumi, professor in ASU’s School of Life Sciences and associate dean in the College of Liberal Arts and Sciences. “We discovered that they turn on at least 326 genes in specific regions of the regenerating tail, including genes involved in embryonic development, response to hormonal signals, and wound healing.”
Other animals, such as salamanders, frog tadpoles, and fish, can also regenerate their tails, with growth mostly at the tip. During tail regeneration, they all turn on genes in what is called the ‘Wnt pathway’ — a process that is required to control stem cells in many organs such as the brain, hair follicles and blood vessel.
However, lizards have a unique pattern of tissue growth that is distributed throughout the tail. It takes lizards more than 60 days to regenerate a functional tail — forming a complex regenerating structure with cells growing into different tissues at a number of sites along the tail.
“Using next-generation technologies to sequence all the genes expressed during regeneration, we have unlocked the mystery of what genes are needed to regrow the lizard tail,” said Kusumi. “By following the genetic recipe for regeneration that is found in lizards, and then harnessing those same genes in human cells, it may be possible to regrow new cartilage, muscle or even spinal cord in the future.”
The researchers also hope their findings will also help repairing birth defects and treating diseases such as arthritis.
Scientists from the University of Arizona College of Medicine, Translational Genomic Research Institute, and Michigan State University where also involved in the research, which was funded by grants from the National Institutes of Health and Arizona Biomedical Research Commission.
Abstract of PLOS ONE paper
Lizards, which are amniote vertebrates like humans, are able to lose and regenerate a functional tail. Understanding the molecular basis of this process would advance regenerative approaches in amniotes, including humans. We have carried out the first transcriptomic analysis of tail regeneration in a lizard, the green anole Anolis carolinensis, which revealed 326 differentially expressed genes activating multiple developmental and repair mechanisms. Specifically, genes involved in wound response, hormonal regulation, musculoskeletal development, and the Wnt and MAPK/FGF pathways were differentially expressed along the regenerating tail axis. Furthermore, we identified 2 microRNA precursor families, 22 unclassified non-coding RNAs, and 3 novel protein-coding genes significantly enriched in the regenerating tail. However, high levels of progenitor/stem cell markers were not observed in any region of the regenerating tail. Furthermore, we observed multiple tissue-type specific clusters of proliferating cells along the regenerating tail, not localized to the tail tip. These findings predict a different mechanism of regeneration in the lizard than the blastema model described in the salamander and the zebrafish, which are anamniote vertebrates. Thus, lizard tail regrowth involves the activation of conserved developmental and wound response pathways, which are potential targets for regenerative medical therapies.