How to capture and convert CO2 from a smokestack in a single step
August 28, 2015
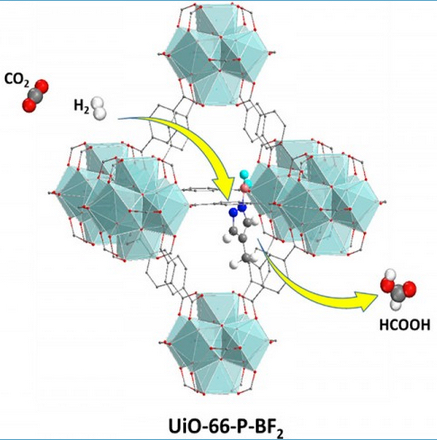
Using a novel catalyst, a single chemical assembly (UiO-66-P-BF2) could capture CO2 and also transform it and hydrogen into formic acid (HCOOH) via a two-step (yellow arrows) reaction (credit: Ye and Johnson/ACS Catalysis)
University of Pittsburgh researchers have invented (in computations) a cheap, efficient catalyst that would capture carbon dioxide (CO2) from coal-burning power plants before it reaches the atmosphere and converts the CO2 into formic acid — a valuable chemical that would create a revenue return.
One current method for capturing CO2 uses Metal–organic frameworks (MOFs), which have a porous, cage-like structure that can absorb CO2, but require expensive catalysts, like platinum.
Instead, the researchers looked for lower-cost non-metallic catalysts, and found that a compound known as UiO-66-PBF2 would do the job.
The method is similar to one developed by Rice University using a combination of amine-rich compounds and carbon-60 molecules.
Those methods both differ from the “diamonds from the sky” approach, which turns CO2 from the air into carbon nanofibers, by capturing the CO2 before it leaves a smokestack. It will be interesting to compare these approaches in terms of CO2 capture efficiency, energy cost, and revenue return.
All three of these methods avoid the costs and energy expenditures from transporting and depositing CO2 at a storage site, required in carbon sequestration.
Abstract of Design of Lewis Pair-Functionalized Metal Organic Frameworks for CO2 Hydrogenation
Efficient catalytic reduction of CO2 is critical for the large-scale utilization of this greenhouse gas. We have used density functional electronic structure methods to design a catalyst for producing formic acid from CO2 and H2 via a two-step pathway having low reaction barriers. The catalyst consists of a microporous metal organic framework that is functionalized with Lewis pair moieties. These functional groups are capable of chemically binding CO2 and heterolytically dissociating H2. Our calculations indicate that the porous framework remains stable after functionalization and chemisorption of CO2 and H2. We have identified a low barrier pathway for simultaneous addition of hydridic and protic hydrogens to carbon and oxygen of CO2, respectively, producing a physisorbed HCOOH product in the pore. We find that activating H2 by dissociative adsorption leads to a much lower energy pathway for hydrogenating CO2 than reacting H2 with chemisorbed CO2. Our calculations provide design strategies for efficient catalysts for CO2 reduction.