How to create 3D mini lungs
March 25, 2015
Scientists coax stem cells to form mini lungs, 3D structures that mimic human lungs and survived in the lab for 100 days (credit: University of Michigan Health System)
Scientists have coaxed stem cells to grow the first three-dimensional human mini lungs, or organoids, to help scientists learn more about lung diseases and test new drugs.
Previous research has focused on deriving lung tissue from flat (2D) cell systems or growing cells onto scaffolds made from donated organs.
“These mini lungs can mimic the responses of real tissues and will be a good model to study how organs form, change with disease, and how they might respond to new drugs,” says senior study author Jason R. Spence, Ph.D., assistant professor of internal medicine and cell and developmental biology at the University of Michigan Medical School.
In an open-access study published in the online journal eLife, the scientists decscribe how they succeeded in growing structures resembling both the large airways known as bronchi and small lung sacs called alveoli.
Since the mini lung structures were developed in a dish, they lack several components of the human lung, including blood vessels, which are a critical component of gas exchange during breathing. But the organoids may still serve as a discovery tool for researchers as they turn basic science ideas into clinical innovations. The idea is to use the 3D structures as a next step up from (or complement to) animal research.
Cell behavior has traditionally been studied in the lab in 2D situations where cells are grown in thin layers on cell-culture dishes. But most cells in the body exist in a three-dimensional environment as part of complex tissues and organs. The advantage of growing 3-D structures of lung tissue, Spence says, is that their organization bears greater similarity to the human lung.
How to make a human lung organoid in a dish
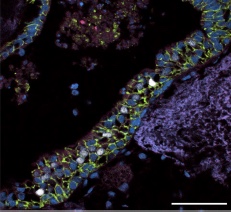
Acellular human lung matrix was seeded with spheroids and cultured for 40 days. Resulting matrices had abundant proximal airway-like structures (scale bar 10 μM) (credit: Briana R. Dye et al./eLife)
To make these lung organoids, researchers at the U-M’s Spence Lab and colleagues from the University of California, San Francisco; Cincinnati Children’s Hospital Medical Center; Seattle Children’s Hospital, and University of Washington manipulated several of the signaling pathways that control the formation of organs:
- Stem cells were instructed to form a type of tissue called endoderm, found in early embryos and that gives rise to the lung, liver and several other internal organs.
- Scientists activated important development pathways that are known to make endoderm form three-dimensional tissue while inhibiting two other key development pathways at the same time.
- The endoderm became tissue that resembles the early lung found in embryos.
- This early lung-like tissue spontaneously formed three-dimensional spherical structures as it developed.
- To make these structures expand and develop into lung tissue, the team exposed the cells to additional proteins that are involved in lung development.
- The resulting lung organoids survived in the lab for more than 100 days.
“We expected different cells types to form, but their organization into structures resembling human airways was a very exciting result,” says lead study author Briana Dye, a graduate student in the U-M Department of Cell and Developmental Biology.
The research is supported by the National Heart, Lung and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the March of Dimes and the U-M’s Center for Organogenesis and Biological Sciences Scholars Program (BSSP).
Abstract of In vitro generation of human pluripotent stem cell derived lung organoids
Recent breakthroughs in 3-dimensional (3D) organoid cultures for many organ systems have led to new physiologically complex in vitro models to study human development and disease. Here, we report the step-wise differentiation of human pluripotent stem cells (hPSCs) (embryonic and induced) into lung organoids. By manipulating developmental signaling pathways hPSCs generate ventral-anterior foregut spheroids, which are then expanded into human lung organoids (HLOs). HLOs consist of epithelial and mesenchymal compartments of the lung, organized with structural features similar to the native lung. HLOs possess upper airway-like epithelium with basal cells and immature ciliated cells surrounded by smooth muscle and myofibroblasts as well as an alveolar-like domain with appropriate cell types. Using RNA-sequencing, we show that HLOs are remarkably similar to human fetal lung based on global transcriptional profiles, suggesting that HLOs are an excellent model to study human lung development, maturation and disease.