How to efficiently convert carbon dioxide from air to methanol fuel
February 3, 2016
Researchers at the University of Southern California (USC) Loker Hydrocarbon Research Institute have created fuel out of thin air — directly converting carbon dioxide from air into methanol at relatively low temperatures for the first time. While methanol can’t currently compete with oil, it will be there when we run out of oil, the researchers note.
The researchers bubbled air through an aqueous solution of pentaethylenehexamine (PEHA), adding a Ru-Macho-BH ruthenium catalyst to encourage hydrogen to latch onto the CO2 under pressure. They then heated the solution, converting 79 percent of the CO2 into methanol.
Though mixed with water, the resulting methanol can be easily distilled, said G.K. Surya Prakash, professor of chemistry and director of the Loker Hydrocarbon Research Institute.
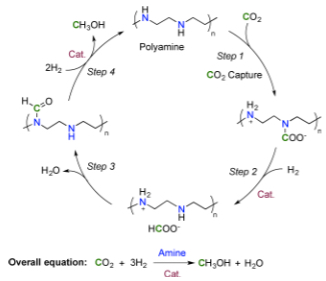
Proposed reaction sequence for CO2 capture and in situ hydrogenation to methanol (credit: J. Kothandaraman et al./Journal of the American Chemical Society)
Scaling up
Methanol (aka “wood alcohol” or “rubbing alcohol”) is attractive because it can be directly used as a clean-burning liquid fuel for internal combustion engines and for fuel cells. It’s also a hydrogen storage medium and a chemical feedstock for producing a myriad of chemicals and products, including ethylene and propylene. It’s one of the most important building blocks in the chemical industry, with an annual production of more than 70 million tons.
The research is part of a broader effort to use renewable energy to transform greenhouse gas into its combustible form — attacking global warming from two angles simultaneously.
Prakash and Olah hope to refine the process to the point that it could be scaled up for industrial use within five to 10 years. “Of course it won’t compete with oil today, at around $30 per barrel,” Prakash said. “But right now we burn fossilized sunshine. We will run out of oil and gas, but the sun will be there for another five billion years. So we need to be better at taking advantage of it as a resource.”
Lower temperatures
Previous efforts have required a slower multistage process with the use of high temperatures and high concentrations of CO2, meaning that renewable energy sources would not be able to efficiently power the process.
The new system operates at around 125 to 165 degrees Celsius (257 to 359 degrees Fahrenheit), minimizing the decomposition of the catalyst — which occurs at 155 degrees Celsius (311 degrees Fahrenheit). The system uses a homogeneous catalyst, making it a quicker “one-pot” process. In a lab, the researchers demonstrated that they were able to run the process five times with only minimal loss of the effectiveness of the catalyst.
The new process was published in the Journal of the American Chemical Society on Dec. 29. The research was supported by the USC Loker Hydrocarbon Research Institute.
Abstract of Conversion of CO2 from Air into Methanol Using a Polyamine and a Homogeneous Ruthenium Catalyst
A highly efficient homogeneous catalyst system for the production of CH3OH from CO2 using pentaethylenehexamine and Ru-Macho-BH (1) at 125–165 °C in an ethereal solvent has been developed (initial turnover frequency = 70 h–1 at 145 °C). Ease of separation of CH3OH is demonstrated by simple distillation from the reaction mixture. The robustness of the catalytic system was shown by recycling the catalyst over five runs without significant loss of activity (turnover number > 2000). Various sources of CO2 can be used for this reaction including air, despite its low CO2 concentration (400 ppm). For the first time, we have demonstrated that CO2 captured from air can be directly converted to CH3OH in 79% yield using a homogeneous catalytic system.