How to explode brain-cancer cells
March 23, 2014
Exploding glioblastoma cancer cells (credit: Cell)
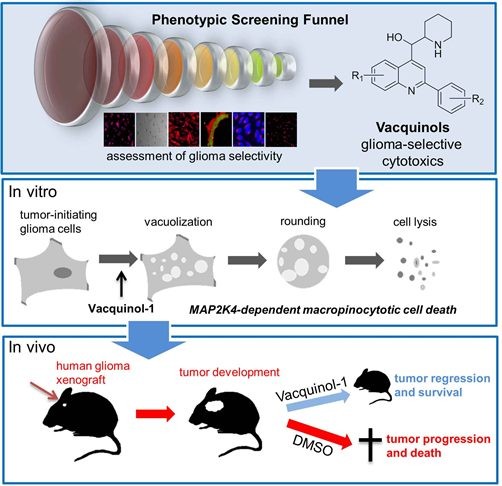
Researchers at Karolinska Institutet and Uppsala University have discovered that a substance called Vacquinol-1 makes cells from glioblastoma, the most aggressive type of brain tumor, literally explode.
When mice were given the substance, which can be given in tablet form, tumor growth was reversed and survival was prolonged. The findings are published in the journal Cell.
The established treatments for glioblastoma are limited, including surgery, radiation, and chemotherapy. The average survival is just 15 months, so it’s critical to find better treatments for malignant brain tumors.
Attacking cancer cells via vacuoles
The researchers transplanted human glioblastoma cells into mice and fed them Vacquinol-1 for five days. The average survival was about 30 days for the control group that did not receive the substance.
Of those who received the substance, six of eight mice were still alive after 80 days. The study was then considered of such interest that the journal Cell wanted to publish the article immediately, said Ernfors.
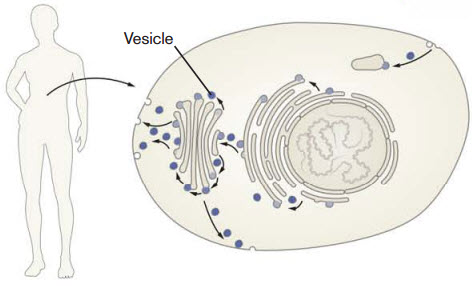
The researchers found that Vacquinol-1 gave the cancer cells uncontrolled vacuolization, a process in which the cell carries substances from outside the cell into its interior.
Exploding cancer cells
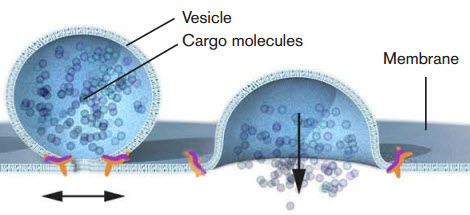
Nobel laureate James E. Rothman discovered that a protein complex (pictured in orange) enables vesicles to fuse with their target membranes. Proteins on the vesicle bind to specific complementary proteins on the target membrane, ensuring that the vesicle fuses at the right location and that cargo molecules are delivered to the correct destination. (Credit: Mattias Karlén/The Nobel Committee for Physiology or Medicine)
This carrying process is accomplished via vacuoles, a type of vesicle. The 2013 Nobel Prize in physiology or medicine was awarded for the discovery of how cellular vesicles move things in cells. When cancer cells were filled with a large amount of vacuoles, the outer wall of the cell collapsed and the cell simply exploded and died.
“This is an entirely new mechanism for cancer treatment, said Patrik Ernfors, professor of tissue biology at the Department of Medical Biochemistry and Biophysics at Karolinska Institutet. A possible medicine based on this principle would therefore attack the glioblastoma in an entirely new way. This principle may also work for other cancer diseases; we have not really explored this yet.”
“We now want to try to take this research discovery through preclinical development and all the way to the clinic. The goal is to get into a phase 1 trial,” he said.
The study has been funded by the Swedish Research Council, the Swedish Cancer Society, the Swedish Foundation for Strategic Research, the Brain Foundation, Hållsten’s Research Foundation, the Torsten Söderberg Foundation, and Wallenberg Scholar.
Abstract of Cell paper
- Glioblastoma cells are vulnerable to vacuolization, ATP depletion, and death
- Vacquinols are potent inducers of vacuolization and cell death
- The MAP kinase MKK4 is required for Vacquinol-induced vacuolization
- Vacquinol-1 has good pharmacokinetics, halts disease, and prolongs mice survival
Glioblastoma multiforme (GBM) is the most aggressive form of brain cancer with marginal life expectancy. Based on the assumption that GBM cells gain functions not necessarily involved in the cancerous process, patient-derived glioblastoma cells (GCs) were screened to identify cellular processes amenable for development of targeted treatments. The quinine-derivative NSC13316 reliably and selectively compromised viability. Synthetic chemical expansion reveals delicate structure-activity relationship and analogs with increased potency, termed Vacquinols. Vacquinols stimulate death by membrane ruffling, cell rounding, massive macropinocytic vacuole accumulation, ATP depletion, and cytoplasmic membrane rupture of GCs. The MAP kinase MKK4, identified by a shRNA screen, represents a critical signaling node. Vacquinol-1 displays excellent in vivo pharmacokinetics and brain exposure, attenuates disease progression, and prolongs survival in a GBM animal model. These results identify a vulnerability to massive vacuolization that can be targeted by small molecules and point to the possible exploitation of this process in the design of anticancer therapies.