How to quickly convert human skin cells into immune-fighting white blood cells
September 17, 2014
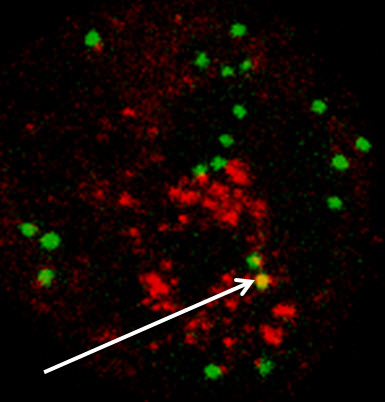
Skin cells converted to white blood cells present functional immunological properties. In the picture, a representative example of a converted cell in which phagocytosis, the process by which certain white blood cells “eat” pathogens, has taken place. Green indicates internalized “beads” used as an in vitro surrogate for bacteria. Red indicates lysosomes, the “digestive” organelle of macrophages. Yellow (highlighted with arrow) indicates targeting of the internalized beads to the lysosomes. (Credit: J. Pulicio et al./Stem Cells)
Salk Institute scientists have turned human skin cells into transplantable white blood cells capable of attacking diseased or cancerous cells or augmenting immune responses against other disorders.
The process, described in the journal Stem Cells, is “quick and safe in mice,” says senior author Juan Carlos Izpisua Belmonte, holder of Salk’s Roger Guillemin Chair.
The existing process, using induced pluripotent stem (iPS) cells to grow new types of cells, requires a long time — at least two months — and tedious laboratory work. Blood cells derived from iPS cells also have other obstacles: an inability to engraft into organs or bone marrow and a likelihood of developing tumors.
The new technique, using indirect lineage conversion, takes just two weeks, engrafts (becomes grafted and functions normally) well, and does not produce tumors.
Inducing cellular memory loss for reprogramming
“We tell skin cells to forget what they are and become what we tell them to be — in this case, white blood cells,” says one of the first authors and Salk researcher Ignacio Sancho-Martinez. “Only two biological molecules are needed to induce such cellular memory loss and to direct a new cell fate.”
Belmonte’s team developed the faster technique and previously demonstrated that these approaches could be used to produce human vascular cells, the ones that line blood vessels. Rather than reversing cells all the way back to a stem cell state before prompting them to turn into something else, such as in the case of iPS cells, the researchers “rewind” skin cells just enough to instruct them to form the more than 200 cell types that constitute the human body.
The technique demonstrated in this study uses a molecule called SOX2 to become somewhat plastic — the stage of losing their “memory” of being a specific cell type. Then, researchers use a genetic factor called miRNA125b that tells the cells that they are actually white blood cells.
The researchers are now conducting toxicology studies and cell transplantation proof-of-concept studies in advance of potential preclinical and clinical studies.
Study co-authors include investigators from the Center of Regenerative Medicine in Barcelona, Spain, and the Centro de Investigacion Biomedica en Red de Enfermedades Raras in Madrid, Spain.
Abstract of Stem Cells paper
Reprogramming technologies have emerged as a promising approach for future regenerative medicine. Here we report on the establishment of a novel methodology allowing for the conversion of human fibroblasts into Hematopoietic Progenitor-like Cells (HPC) with macrophage differentiation potential. SOX2 overexpression in human fibroblasts, a gene found to be upregulated during hematopoietic reconstitution in mice, induced the rapid appearance of CD34+ cells with a concomitant upregulation of mesoderm-related markers. Profiling of Cord Blood hematopoietic progenitor cell populations identified miR-125b as a factor facilitating commitment of SOX2-generated CD34+ cells to immature hematopoietic-like progenitor cells with grafting potential. Further differentiation towards the monocytic lineage resulted in the appearance of CD14+ cells with functional phagocytic capacity. In vivo transplantation of SOX2/miR-125b-generated CD34+ cells facilitated the maturation of the engrafted cells towards CD45+ cells and ultimately the monocytic/macrophage lineage. Altogether, our results indicate that strategies combining lineage conversion and further lineage specification by in vivo or in vitro approaches could help to circumvent long-standing obstacles for the reprogramming of human cells into hematopoietic cells with clinical potential.