How to smuggle killer drugs into cancer cells
May 13, 2014
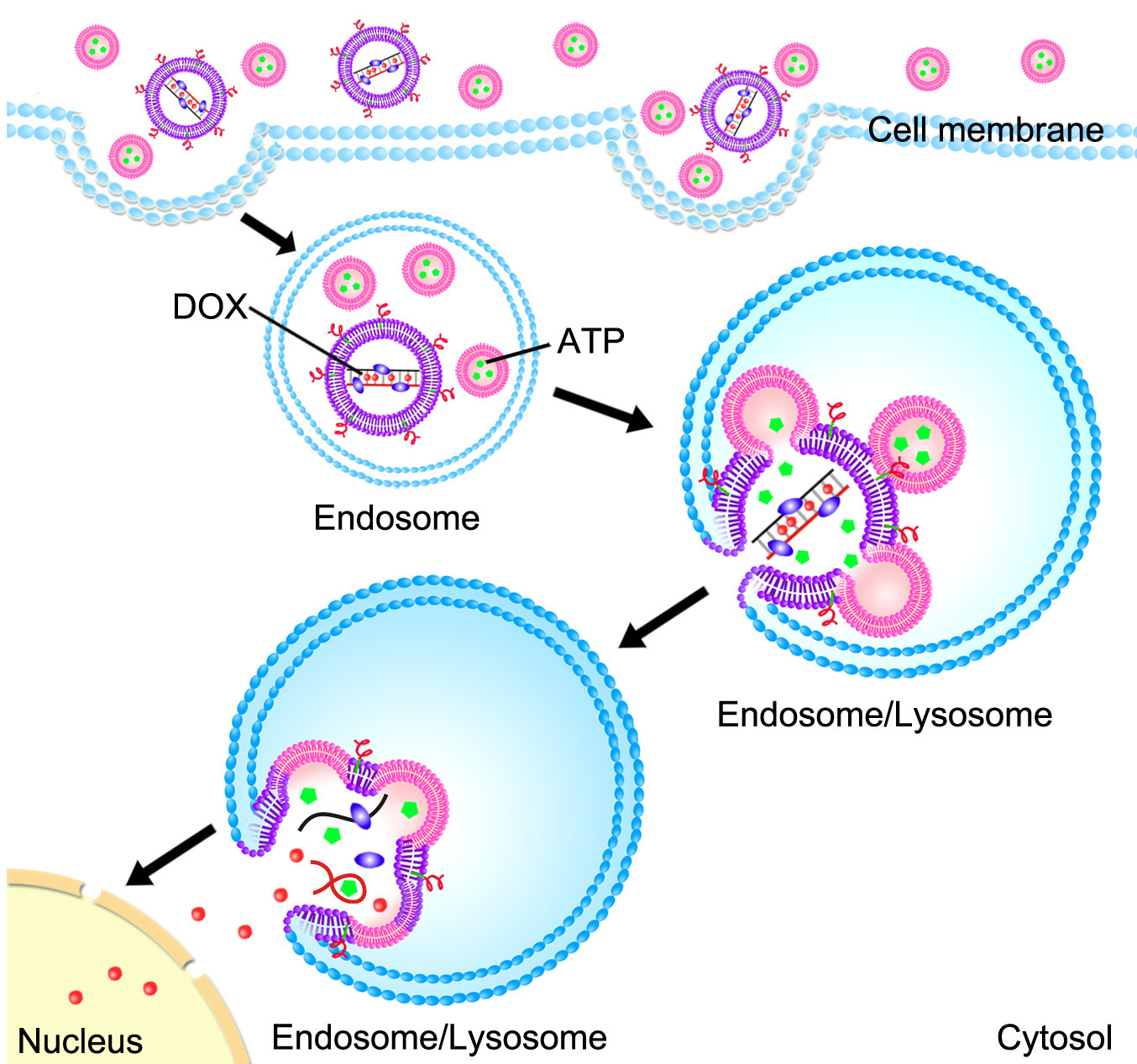
Smuggling a drug (DOX) into a cancer cell (credit: Ran Mo)
Biomedical engineering researchers have developed an anti-cancer drug-delivery method that essentially smuggles the drug into a cancer cell before triggering its release.
“This is an efficient, fast-acting way of delivering drugs to cancer cells and triggering cell death,” says Dr. Ran Mo, lead author of a paper on the work and a postdoctoral researcher in the joint biomedical engineering program at North Carolina State University and the University of North Carolina at Chapel Hill.
“We also used lipid-based nanocapsules that are already in use for clinical applications, making it closer to use in the real world.”
The technique uses nanoscale lipid-based capsules (liposomes) to deliver both the hidden doxorubicin (Dox) drug (used in cancer chemotherapy) and release mechanism into cancer cells.*
In a mouse model, the researchers found that the new technique significantly decreased the size of breast cancer tumors compared to treatment that used Dox without the nanoscale liposomes.
“This is a novel drug delivery design,” Dr. Zhen Gu, senior author of the paper and an assistant professor in the joint biomedical engineering program, explained to KurzweilAI. “It is the first time to apply fusion of liposomes and ATP responsive complex together to achieve precise release of anticancer drugs inside cells.”
Availability of clinical use “may take a few years,” he said, “since we will perform large-animal studies before clinical trials.”
Their paper is published in Angewandte Chemie. The research was supported by the National Institutes of Health and funding from NC State and UNC-Chapel Hill.
* One set of liposomes contains adenosine-5’-triphosphate (ATP), the “energy molecule.” A second set of liposomes contains an anti-cancer drug called doxorubicin (Dox) that is embedded in a complex of DNA molecules.
As the liposomes are absorbed into a cancer cell, they are sealed off from the rest of the cell in an endosome — a compartment that walls off all foreign material that gets into a cell. The environment inside an endosome is acidic, which causes the Dox liposomes and ATP liposomes to fuse together, as well as to the wall of the endosome itself.
Meanwhile, two other things are happening simultaneously. First, the ATP liposomes spill their ATP into the Dox liposomes, releasing the Dox from its DNA cage. Second, the walls of the Dox liposomes create an opening in the endosome, spilling their Dox-rich contents into the surrounding cell — leading to cell death.
Abstract of Angewandte Chemie paper
A liposome-based co-delivery system composed of a fusogenic liposome encapsulating ATP-responsive elements with chemotherapeutics and a liposome containing ATP was developed for ATP-mediated drug release triggered by liposomal fusion. The fusogenic liposome had a protein–DNA complex core containing an ATP-responsive DNA scaffold with doxorubicin (DOX) and could release DOX through a conformational change from the duplex to the aptamer/ATP complex in the presence of ATP. A cell-penetrating peptide-modified fusogenic liposomal membrane was coated on the core, which had an acid-triggered fusogenic potential with the ATP-loaded liposomes or endosomes/lysosomes. Directly delivering extrinsic liposomal ATP promoted the drug release from the fusogenic liposome in the acidic intracellular compartments upon a pH-sensitive membrane fusion and anticancer efficacy was enhanced both in vitro and in vivo.