How to trigger self-powered mechanical movement
March 3, 2016
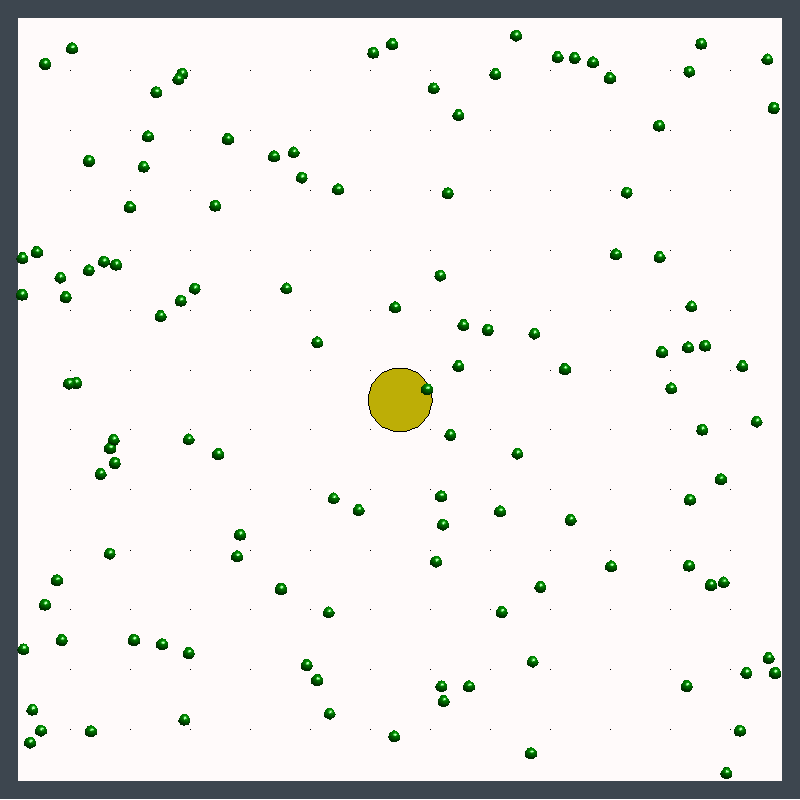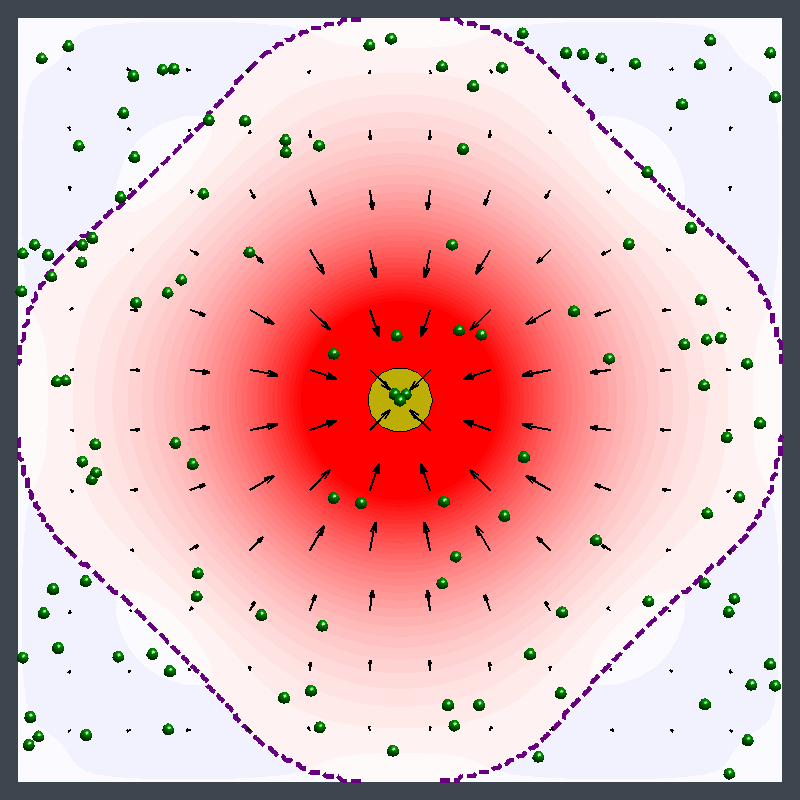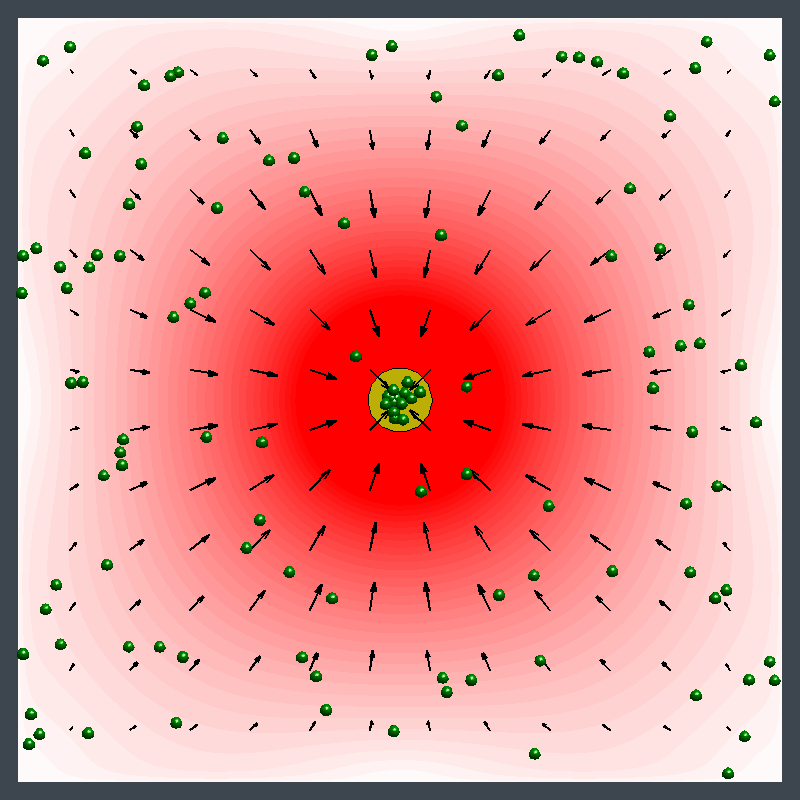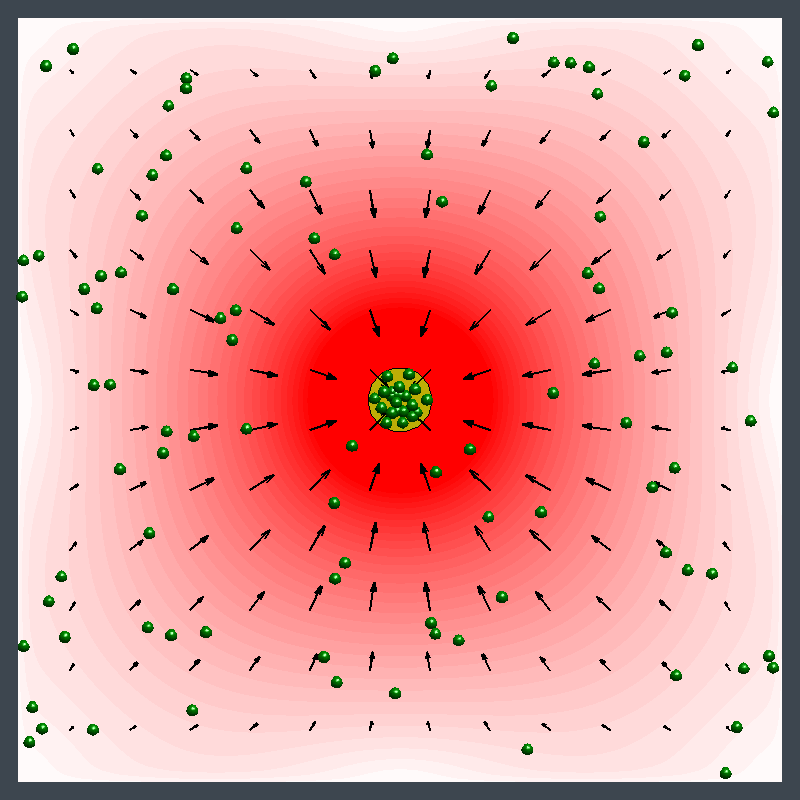
This animation illustrates an enzyme pump pushing particles away, then drawing them in. Initially, the flow pushes particles away from the pump (in the blue region). Later, the flow direction reverses and draws particles toward the pump (in the red region). (credit: University of Pittsburgh)
A new way to use the chemical reactions of certain enzymes to trigger self-powered mechanical movement has been developed by a team of researchers at Penn State University and the University of Pittsburgh.
These enzyme micropumps could be used for detecting substances, moving particles to build small structures, and delivering medications.
“One potential use is the release of insulin to a diabetes patient from a reservoir at a rate proportional to the concentration of glucose in the person’s blood,” said Ayusman Sen, Distinguished Professor of Chemistry at Penn State. “Another example is an enzyme pump that is triggered by nerve toxins to release an antidote agent to decontaminate and treat an exposed person.
The pumps provide precise control over flow rate without the aid of an external power source and are capable of turning on in response to specific chemicals in solution.
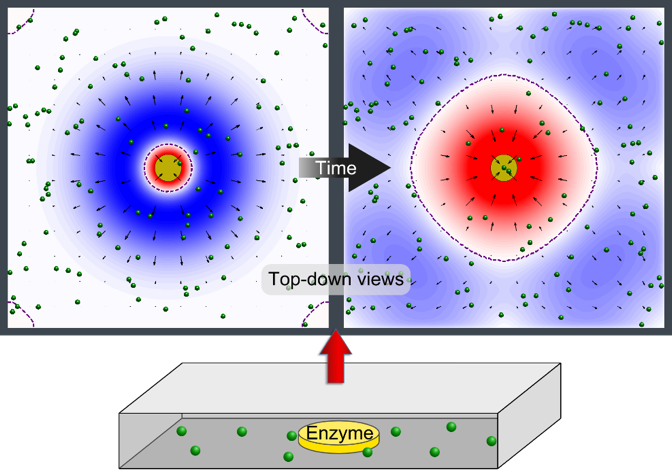
This image illustrates pumping in two directions at once with an enzyme patch. A patch of enzymes immobilized on a surface acts as a fluid pump. The fluid, and the small particles (green spheres) carried by the fluid, can simultaneously be pumped away from the patch (blue) in some parts of the chamber and toward the patch (red) in other locations. This behavior changes over time and is due to the changes in fluid density that the reaction produces. (credit: University of Pittsburgh)
A paper describing the team’s research was published last week in the journal Proceedings of the National Academy of Sciences. The team’s research was supported by the Charles E. Kauffman Foundation, the National Science Foundation, and the Defense Threat Reduction Agency.
Absract of Convective flow reversal in self-powered enzyme micropumps
Surface-bound enzymes can act as pumps that drive large-scale fluid flows in the presence of their substrates or promoters. Thus, enzymatic catalysis can be harnessed for “on demand” pumping in nano- and microfluidic devices powered by an intrinsic energy source. The mechanisms controlling the pumping have not, however, been completely elucidated. Herein, we combine theory and experiments to demonstrate a previously unreported spatiotemporal variation in pumping behavior in urease-based pumps and uncover the mechanisms behind these dynamics. We developed a theoretical model for the transduction of chemical energy into mechanical fluid flow in these systems, capturing buoyancy effects due to the solution containing nonuniform concentrations of substrate and product. We find that the qualitative features of the flow depend on the ratios of diffusivities δ=DP=DS and expansion coefficients β=βP=βS of the reaction substrate (S) and product (P). If δ>1 and δ>β (or if δ<1 and δ<β), an unexpected phenomenon arises: the flow direction reverses with time and distance from the pump. Our experimental results are in qualitative agreement with the model and show that both the speed and direction of fluid pumping (i) depend on the enzyme activity and coverage, (ii) vary with the distance from the pump, and (iii) evolve with time. These findings permit the rational design of enzymatic pumps that accurately control the direction and speed of fluid flow without external power sources, enabling effective, self-powered fluidic devices.