Why carbon-nanotube fibers make ideal implantable brain electrodes
March 26, 2015
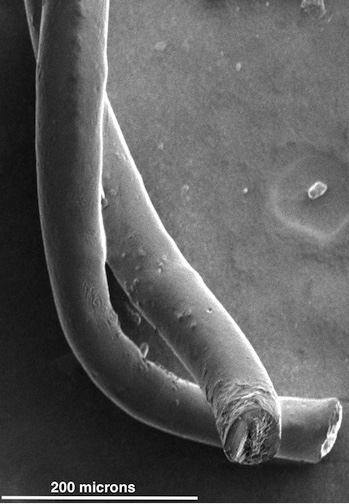
Pairs of carbon nanotube fibers have been tested for potential use as implantable electrodes to treat patients with neurological disorders like Parkinson’s disease. The fibers invented at Rice University proved to be far better than the metallic wires now used to stimulate neurons in the brain. (credit: the Pasquali Lab)
Rice University scientists have found that the carbon nanotube fibers they developed for aerospace are superior to metal and plain-carbon electrodes for deep brain stimulation for neurological disorders such as Parkinson’s and for brain-machine interfaces to neural circuits in the brain.
The individual nanotubes measure only a few nanometers across, but when millions are bundled in a process called wet spinning, they become thread-like fibers about a quarter the width of a human hair.
Strong as metal but soft as silk and highly conductive
“We developed these fibers as high-strength, high-conductivity materials” for aerospace applications, where strength, weight and conductivity are paramount, said co-developer Matteo Pasquali, a chemist and chemical engineer.
“Yet, once we had them in our hand, we realized that they had an unexpected property: They are really soft, much like a thread of silk. Their unique combination of strength, conductivity and softness makes them ideal for interfacing with the electrical function of the human body.”
Pudding-compatible design may revolutionize brain implants
“The brain is basically the consistency of pudding and doesn’t interact well with stiff metal electrodes,” said Caleb Kemere, a Rice assistant professor who brought expertise in animal models of Parkinson’s disease. “The dream is to have electrodes with the same consistency [as the brain], and that’s why we’re really excited about these flexible carbon nanotube fibers and their long-term biocompatibility.”
Tests on cells and then in rats with Parkinson’s symptoms proved several advantages for the fibers:
- They are stable and as efficient as commercial platinum electrodes at only a fraction of the thickness.
- The soft fibers caused little inflammation, which helped maintain strong electrical connections to neurons by preventing the body’s defenses from scarring and encapsulating the site of the injury.
- The highly conductive carbon nanotube fibers show much more favorable impedance (similar to resistance) than state-of-the-art metal electrodes, allowing for use of lower voltages.
- The working end of the fiber is the exposed tip, which is about the width of a neuron. The rest is encased with a three-micron layer of a flexible, biocompatible polymer with excellent insulating properties.
- Doctors who implant deep brain stimulation devices start with a recording probe able to “listen” to neurons that emit characteristic signals depending on their functions, Kemere said. Once a surgeon finds the right spot, the probe is removed and the stimulating electrode gently inserted, but it’s “too big to detect any spiking activity, so basically the clinical devices send continuous pulses regardless of the response of the brain.” The new carbon nanotube fibers simplify implantation because they can do both functions (send and receive signals),
Kemere foresees a closed-loop system that can read neuronal signals and adapt stimulation therapy in real time. He anticipates building a device with many electrodes that can be addressed individually to gain fine control over stimulation and monitoring from a small, implantable device.
The Welch Foundation, the National Science Foundation, and the Air Force Office of Scientific Research supported the research.
Abstract of Neural Stimulation and Recording with Bidirectional, Soft Carbon Nanotube Fiber Microelectrodes
The development of microelectrodes capable of safely stimulating and recording neural activity is a critical step in the design of many prosthetic devices, brain machine interfaces and therapies for neurologic or nervous-system-mediated disorders. Metal electrodes are inadequate prospects for the miniaturization needed to attain neuronal-scale stimulation and recording because of their poor electrochemical properties, high stiffness and propensity to fail due to bending fatigue. Here we demonstrate neural recording and stimulation using carbon nanotube (CNT) fiber electrodes. In vitro characterization shows that the tissue contact impedance of CNT fibers is remarkably lower than state-of-the-art metal electrodes, making them suitable for recording single neuron activity without additional surface treatments. In vivo chronic studies in parkinsonian rodents show that CNT fiber microelectrodes stimulate neurons as effectively as metal electrodes with ten times larger surface area, while eliciting a significantly reduced inflammatory response. The same CNT fiber microelectrodes can record neural activity for weeks, paving the way for the development of novel multifunctional, dynamic neural interfaces with long-term stability.