IBM Research discovers new class of industrial polymers with exceptional properties
May 16, 2014

A scanning electron microscopy (SEM) image of the new ultra-strong polymer reinforced with carbon nanotubes (credit: IBM)
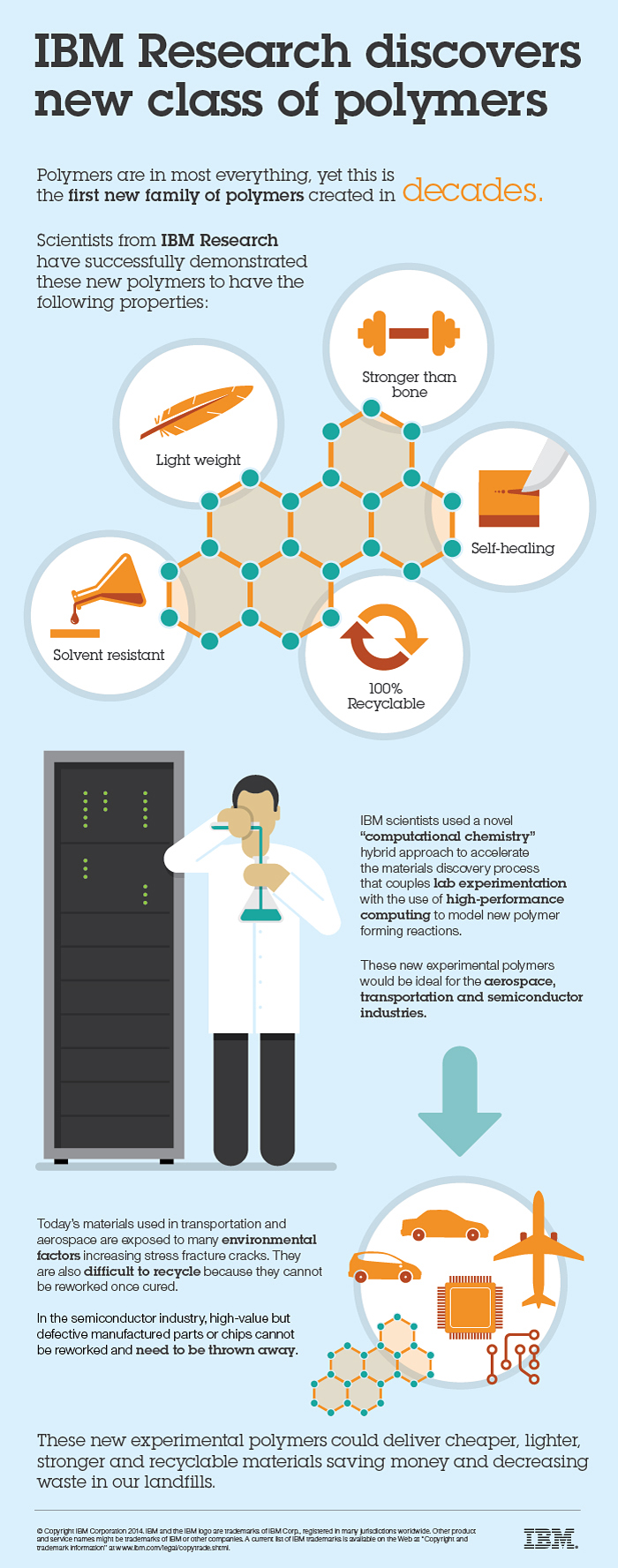
IBM Research scientists have discovered a new class of polymer materials that demonstrate resistance to cracking, strength higher than bone, and the ability to reform to their original shape (self-heal) and original material.
These materials can also be transformed into new polymer structures to further bolster their strength by 50%, making them ultra-strong and lightweight, and could result in cheaper, lighter, stronger and recyclable materials.
The finding combines a number of attributes, each achieved in separate studies by various researchers, as reported by KurzweilAI.
The discovery could transform manufacturing and fabrication in the fields of transportation, aerospace, and microelectronics, the scientists say.
This research was published in the journal Science, with collaborators from UC Berkeley, Eindhoven University of Technology, and King Abdulaziz City for Science and Technology (KACST), Saudi Arabia.
(Credit: IBM)
Limitations of current polymers
Polymers, a long chain of molecules that are connected through chemical bonds, are an indispensable part of everyday life. They are a core material in common items ranging from clothing and drink bottles (polyesters), paints (polyacrylics), plastic milk bottles (polyethylene), secure food packaging (polyolefins, polystyrene) to major parts of cars and planes (epoxies, polyamides and polyimides).
However, today’s polymer materials are limited in various ways. In transportation and aerospace, structural components or composites are exposed to many environmental factors (de-icing of planes, exposure to fuels, cleaning products, etc.) and exhibit poor environmental-stress crack resistance (such as catastrophic failure upon exposure to a solvent).
Also, these polymers are difficult to recycle because they cannot be remolded or reworked once cured or thermally decomposed by heating to high temperatures. As a result, these end up in the landfill together with toxins such as plasticizers, fillers, and color additives, which are not biodegradable.
IBM has discovered two new related classes of materials that possess a very distinctive range of properties that include high stiffness, solvent resistance, the ability to heal themselves once a crack is introduced and to be used as a resin for filled composite materials to further bolster their strength.
The ability to selectively recycle a structural component would have significant impact in the semiconductor industry, advanced manufacturing or advanced composites for transportation, as one would be able to rework high-value but defective manufactured parts or chips instead of throwing them away. This could bolster fabrication yields, save money and significantly decrease microelectronic waste.
How it Works
This polymer remains intact when it is exposed to basic water (high pH), but selectively decomposes when exposed to very acidic water (very low pH). This means that under the right conditions, this polymer can be reverted back to its starting materials, which enables it for reuse for other polymers.
The material can also be manufactured to have even higher strength if carbon nanotubes or other reinforcing fillers are mixed into the polymer and are heated to high temperatures. This process enables polymers to have properties similar to metals. An advantage to using polymers in this case over metals is that they are more lightweight, which in the transportation industry translates to savings in fuel costs.
At low temperatures (just over room temperature), another type of polymer can be formed into elastic gels that are still stronger than most polymers, but still maintains its flexibility because of solvent that is trapped within the network, stretching like a rubber band.
Probably the most unexpected and remarkable characteristic of these gels is that if they are severed and the pieces are placed back in proximity so they physically touch, the chemical bonds are reformed between the pieces making it a single unit again within seconds. This type of polymer is called a “self healing” polymer because of its ability to do this and is made possible here due to hydrogen-bonding interactions in the hemiaminal polymer network.
One could envision using these types of materials as adhesives or mixing in with other polymers to induce self-healing properties in the polymer mixture. Furthermore, these polymers are reversible constructs, which means they can be recycled in neutral water, and might find use in applications that require reversible assemblies, such as drug cargo delivery.
IBM scientists used a novel “computational chemistry” hybrid approach to accelerate the materials discovery process that couples lab experimentation with the use of high-performance computing to model new polymer forming reactions. The unconventional method is a departure from traditional techniques and led to the identification of several previously undiscovered classes of polymers in what was believed to be an established area of materials science researched extensively since the 1950s.
IBM Research’s computational chemistry efforts can take out a lot of the guesswork and accelerate a whole new range of potential applications from developing a disease-specific drugs or cheap, light, tough and completely recyclable panels on a car, the scientists say.
The first new class of polymers discovered in over 20 years by IBM researchers is the world’s first family of materials that are stronger than bone, self-healing and solvent-resistant while being completely recyclable back to their starting material. These new experimental polymers, coming by way of a novel ‘computational chemistry’ hybrid approach to accelerate the materials discovery process, could deliver cheaper, lighter, stronger and recyclable materials ideal for the semiconductor, aerospace, airline and automotive industries.
Abstract of Science paper
Nitrogen-based thermoset polymers have many industrial applications (for example, in composites), but are difficult to recycle or rework. We report a simple one-pot, low-temperature polycondensation between paraformaldehyde and 4,4ʹ-oxydianiline (ODA) that forms hemiaminal dynamic covalent networks (HDCNs), which can further cyclize at high temperatures, producing poly(hexahydrotriazine)s (PHTs). Both materials are strong thermosetting polymers, and the PHTs exhibited very high Young’s moduli (up to ~14.0 gigapascals and up to 20 gigapascals when reinforced with surface-treated carbon nanotubes), excellent solvent resistance, and resistance to environmental stress cracking. However, both HDCNs and PHTs could be digested at low pH (<2) to recover the bisaniline monomers. By simply using different diamine monomers, the HDCN- and PHT-forming reactions afford extremely versatile materials platforms. For example, when poly(ethylene glycol) (PEG) diamine monomers were used to form HDCNs, elastic organogels formed that exhibited self-healing properties.