Just add water: a portable hydrogen fuel cell
January 25, 2013
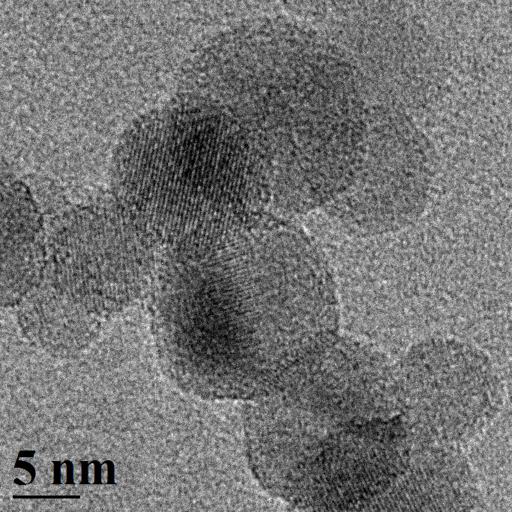
A close-up of spherical silicon nanoparticles about 10 nanometers in diameter. In Nano Letters, UB scientists report that these particles could form the basis of new technologies that generate hydrogen for portable power applications. (Credit: Swihart Research Group/University at Buffalo)
Battery dead in the middle of a phone call and you left your charger home, or worse, you’re on a camping trip. Sound familiar?
No prob, just grab some nanosilicon powder, mix with water, and zap: instant hydrogen fuel to generate recharge current — thanks to University at Buffalo researchers, who have discovered that super-small particles of silicon react with water to produce hydrogen almost instantaneously.
They created spherical silicon particles about 10 nanometers in diameter. When combined with water, these particles reacted to form silicic acid (a nontoxic byproduct) and hydrogen — a potential source of energy for fuel cells.
The reaction didn’t require any light, heat or electricity, and also created hydrogen about 150 times faster than similar reactions using silicon particles 100 nanometers wide, and 1,000 times faster than bulk silicon.
Just add water
It takes significant energy and resources to produce the super-small silicon balls, but the particles could help power portable devices in situations where water is available and portability is more important than low cost, the researchers said.
Military operations and camping trips are two examples of such scenarios.
You’ll take along a small hydrogen fuel cell and some plastic cartridges of silicon nanopowder mixed with an activator. Out of juice? Just add water, advises Mark T. Swihart, UB professor of chemical and biological engineering.